Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study
- PMID: 25638521
- PMCID: PMC4374102
- DOI: 10.1016/S1473-3099(14)71060-6
Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study
Abstract
Background: Rotavirus is the main cause of severe acute gastroenteritis in children in Africa. Monovalent human rotavirus vaccine (RV1) was added into Malawi's infant immunisation schedule on Oct 29, 2012. We aimed to assess the impact and effectiveness of RV1 on rotavirus gastroenteritis in the 2 years after introduction.
Methods: From Jan 1, 2012, to June 30, 2014, we recruited children younger than 5 years who were admitted into Queen Elizabeth Central Hospital, Blantyre, Malawi, with acute gastroenteritis. We assessed stool samples from these children for presence of rotavirus with use of ELISA and we genotyped rotaviruses with use of RT-PCR. We compared rotavirus detection rates in stool samples and incidence of hospital admittance for rotavirus in children from Jan 1 to June 30, in the year before vaccination (2012) with the same months in the 2 years after vaccination was introduced (2013 and 2014). In the case-control portion of our study, we recruited eligible rotavirus-positive children from the surveillance platform and calculated vaccine effectiveness (one minus the odds ratio of vaccination) by comparing infants with rotavirus gastroenteritis with infants who tested negative for rotavirus, and with community age-matched and neighbourhood-matched controls.
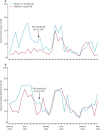
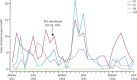
Findings: We enrolled 1431 children, from whom we obtained 1417 stool samples (99%). We detected rotavirus in 79 of 157 infants (50%) before the vaccine, compared with 57 of 219 (40%) and 52 of 170 (31%) in successive calendar years after vaccine introduction (p=0·0002). In the first half of 2012, incidence of rotavirus hospital admission was 269 per 100,000 infants compared with 284 in the same months of 2013 (rise of 5·8%, 95% CI -23·1 to 45·4; p=0·73) and 153 in these months in 2014 (a reduction from the prevaccine period of 43·2%, 18·0-60·7; p=0·003). We recruited 118 vaccine-eligible rotavirus cases (median age 8·9 months; IQR 6·6-11·1), 317 rotavirus-test-negative controls (9·4 months; 6·9-11·9), and 380 community controls (8·8 months; 6·5-11·1). Vaccine effectiveness for two doses of RV1 in rotavirus-negative individuals was 64% (95% CI 24-83) and community controls was 63% (23-83). The point estimate of effectiveness was higher against genotype G1 than against G2 and G12.
Interpretation: Routine use of RV1 reduced hospital admissions for several genotypes of rotavirus in children younger than 5 years, especially in infants younger than 1 year. Our data support introduction of rotavirus vaccination at the WHO recommended schedule, with continuing surveillance in high-mortality countries.
Funding: Wellcome Trust, GlaxoSmithKline Biologicals.
Copyright © 2015 Bar-Zeev, et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Rotavirus vaccines roll-out in resource-deprived regions.Lancet Infect Dis. 2015 Apr;15(4):368-70. doi: 10.1016/S1473-3099(14)71089-8. Epub 2015 Jan 29. Lancet Infect Dis. 2015. PMID: 25638520 No abstract available.
References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, for the WHO-coordinated Global Rotavirus Surveillance Network 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. - PubMed
-
- Richardson V, Hernandez-Pichardo J, Quintanar-Solares M. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010;362:299–305. - PubMed
-
- Cortese MM, Tate JE, Simonsen L, Edelman L, Parashar UD. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J. 2010;29:489–494. - PubMed
-
- Gurgel RG, Bohland AK, Vieira SC. Incidence of rotavirus and all-cause diarrhea in northeast Brazil following the introduction of a national vaccination program. Gastroenterology. 2009;137:1970–1975. - PubMed
-
- Molto Y, Cortes JE, De Oliveira LH. Reduction of diarrhea-associated hospitalizations among children aged <5 years in Panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J. 2011;30:S16–S20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

