Nicotinic ACh receptors as therapeutic targets in CNS disorders
- PMID: 25639674
- PMCID: PMC4324614
- DOI: 10.1016/j.tips.2014.12.002
Nicotinic ACh receptors as therapeutic targets in CNS disorders
Abstract
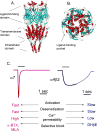
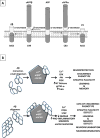
The neurotransmitter acetylcholine (ACh) can regulate neuronal excitability by acting on the cys-loop cation-conducting ligand-gated nicotinic ACh receptor (nAChR) channels. These receptors are widely distributed throughout the central nervous system (CNS), being expressed on neurons and non-neuronal cells, where they participate in a variety of physiological responses such as anxiety, the central processing of pain, food intake, nicotine seeking behavior, and cognitive functions. In the mammalian brain, nine different subunits have been found thus far, which assemble into pentameric complexes with much subunit diversity; however, the α7 and α4β2 subtypes predominate in the CNS. Neuronal nAChR dysfunction is involved in the pathophysiology of many neurological disorders. Here we will briefly discuss the functional makeup and expression of the nAChRs in mammalian brain, and their role as targets in neurodegenerative diseases (in particular Alzheimer's disease, AD), neurodevelopmental disorders (in particular autism and schizophrenia), and neuropathic pain.
Keywords: cys-loop receptor; nAChR; α4β2 receptor; α7 receptor.
Published by Elsevier Ltd.
Figures
References
-
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

