Improving the photocatalytic reduction of CO2 to CO through immobilisation of a molecular Re catalyst on TiO2
- PMID: 25639778
- PMCID: PMC4471553
- DOI: 10.1002/chem.201405041
Improving the photocatalytic reduction of CO2 to CO through immobilisation of a molecular Re catalyst on TiO2
Abstract
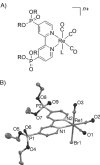
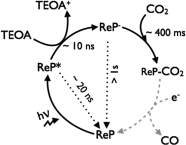
The photocatalytic activity of phosphonated Re complexes, [Re(2,2'-bipyridine-4,4'-bisphosphonic acid) (CO)3(L)] (ReP; L = 3-picoline or bromide) immobilised on TiO2 nanoparticles is reported. The heterogenised Re catalyst on the semiconductor, ReP-TiO2 hybrid, displays an improvement in CO2 reduction photocatalysis. A high turnover number (TON) of 48 molCO molRe(-1) is observed in DMF with the electron donor triethanolamine at λ>420 nm. ReP-TiO2 compares favourably to previously reported homogeneous systems and is the highest TON reported to date for a CO2-reducing Re photocatalyst under visible light irradiation. Photocatalytic CO2 reduction is even observed with ReP-TiO2 at wavelengths of λ>495 nm. Infrared and X-ray photoelectron spectroscopies confirm that an intact ReP catalyst is present on the TiO2 surface before and during catalysis. Transient absorption spectroscopy suggests that the high activity upon heterogenisation is due to an increase in the lifetime of the immobilised anionic Re intermediate (t50% >1 s for ReP-TiO2 compared with t50% = 60 ms for ReP in solution) and immobilisation might also reduce the formation of inactive Re dimers. This study demonstrates that the activity of a homogeneous photocatalyst can be improved through immobilisation on a metal oxide surface by favourably modifying its photochemical kinetics.
Keywords: CO2 reduction; heterogeneous catalysis; immobilisation; photocatalysis; time-resolved spectroscopy.
© 2015 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

