Diverse roles of STING-dependent signaling on the development of cancer
- PMID: 25639870
- PMCID: PMC4998969
- DOI: 10.1038/onc.2014.457
Diverse roles of STING-dependent signaling on the development of cancer
Abstract
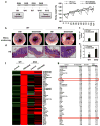
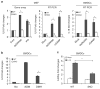
Stimulator of interferon genes (STING) is a cellular sensor that controls cytosolic DNA-activated innate immune signaling. We have previously demonstrated that STING-deficient mice are resistant to carcinogen-induced skin cancer, similar to myeloid differentiation primary response gene 88 (MyD88) deficient mice, since the production of STING-dependent DNA-damage-induced proinflammatory cytokines, that likely require MyD88 signaling to exert their growth-promoting activity, are prevented. In contrast, MyD88-deficient mice are sensitive to colitis-associated cancer (CAC), since selected cytokines generated following DNA-damage also activate repair pathways, which can help prevent tumor development. Here, we demonstrate that STING signaling facilitates wound repair processes and that analogous to MyD88-deficient mice, STING-deficient mice (SKO) are prone to CAC induced by DNA-damaging agents. SKO mice harboring tumors exhibited low levels of tumor-suppressive interleukin-22 binding protein (IL-22BP) compared to normal mice, a cytokine considered critical for preventing colon-related cancer. Our data indicate that STING constitutes a critical component of the host early response to intestinal damage and is essential for invigorating tissue repair pathways that may help prevent tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

