Tunable nanostructured coating for the capture and selective release of viable circulating tumor cells
- PMID: 25640006
- PMCID: PMC4492283
- DOI: 10.1002/adma.201404677
Tunable nanostructured coating for the capture and selective release of viable circulating tumor cells
Abstract
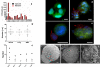
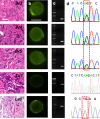
A layer-by-layer gelatin nanocoating is presented for use as a tunable, dual response biomaterial for the capture and release of circulating tumor cells (CTCs) from cancer patient blood. The entire nanocoating can be dissolved from the surface of microfluidic devices through biologically compatible temperature shifts. Alternatively, individual CTCs can be released through locally applied mechanical stress.
Keywords: circulating tumor cells; circulating tumor clusters; microfluidics; nanostructured coatings; single cell sequencing.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LWMM, Hayes DF. New Engl. J. Med. 2004;351:781. - PubMed
-
- Powell AA, Talasaz AH, Zhang H, Coram MA, Reddy A, Deng G, Telli ML, Advani RH, Carlson RW, Mollick JA, Sheth S, Kurian AW, Ford JM, Jeffrey SS. Plos One. 2012;7:33788. - PMC - PubMed
- Ozkumur E, Shah AM, Ciciliano JC, Emmink BL, Miyamoto DT, Brachtel E, Yu M, Chen P.-i., Morgan B, Trautwein J, Kimura A, Sengupta S, Stott SL, Karabacak NM, Barber TA, Walsh JR, Smith K, Spuhler PS, Sullivan JP, Lee RJ, Ting DT, Luo X, Shaw AT, Bardia A, Sequist LV, Louis DN, Maheswaran S, Kapur R, Haber DA, Toner M. Sci. Transl. Med. 2013;5:179ra47. 1. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

