Pulsed focused ultrasound pretreatment improves mesenchymal stromal cell efficacy in preventing and rescuing established acute kidney injury in mice
- PMID: 25640064
- PMCID: PMC4376574
- DOI: 10.1002/stem.1965
Pulsed focused ultrasound pretreatment improves mesenchymal stromal cell efficacy in preventing and rescuing established acute kidney injury in mice
Erratum in
-
Corrigendum.Stem Cells. 2020 Mar;38(3):464. doi: 10.1002/stem.3149. Epub 2020 Jan 24. Stem Cells. 2020. PMID: 31976591 No abstract available.
Abstract
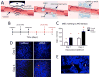
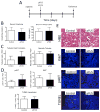
Animal studies have shown that mesenchymal stromal cell (MSC) infusions improve acute kidney injury (AKI) outcomes when administered early after ischemic/reperfusion injury or within 24 hours after cisplatin administration. These findings have spurred several human clinical trials to prevent AKI. However, no specific therapy effectively treats clinically obvious AKI or rescues renal function once advanced injury is established. We investigated if noninvasive image-guided pulsed focused ultrasound (pFUS) could alter the kidney microenvironment to enhance homing of subsequently infused MSC. To examine the efficacy of pFUS-enhanced cell homing in disease, we targeted pFUS to kidneys to enhance MSC homing after cisplatin-induced AKI. We found that pFUS enhanced MSC homing at 1 day post-cisplatin, prior to renal functional deficits, and that enhanced homing improved outcomes of renal function, tubular cell death, and regeneration at 5 days post-cisplatin compared to MSC alone. We then investigated whether pFUS+MSC therapy could rescue established AKI. MSC alone at 3 days post-cisplatin, after renal functional deficits were obvious, significantly improved 7-day survival of animals. Survival was further improved by pFUS and MSC. pFUS prior to MSC injections increased IL-10 production by MSC that homed to kidneys and generated an anti-inflammatory immune cell profile in treated kidneys. This study shows pFUS is a neoadjuvant approach to improve MSC homing to diseased organs. pFUS with MSC better prevents AKI than MSC alone and allows rescue therapy in established AKI, which currently has no meaningful therapeutic options.
Keywords: Acute kidney injury; Cellular therapy; Cisplatin; Kidney; Mesenchymal stromal cells; Renal; Renal failure; Stem cell transplantation.
© 2015 AlphaMed Press.
Figures





References
-
- Chertow GM, Soroko SH, Paganini EP, et al. Mortality after acute renal failure: models for prognostic stratification and risk adjustment. Kidney Int. 2006;70:1120–1126. - PubMed
-
- Li PK, Burdmann EA, Mehta RL. Acute kidney injury: Acute kidney injury--global health alert. Nat Rev Nephrol. 2013;9:133–135. - PubMed
-
- Liangos O, Wald R, O’Bell JW, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. - PubMed
-
- Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. - PubMed
-
- Allgren RL, Marbury TC, Rahman SN, et al. Anaritide in acute tubular necrosis. Auriculin Anaritide Acute Renal Failure Study Group. N Engl J Med. 1997;336:828–834. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

