Generation of highly enriched populations of optic vesicle-like retinal cells from human pluripotent stem cells
- PMID: 25640818
- PMCID: PMC4353653
- DOI: 10.1002/9780470151808.sc01h08s32
Generation of highly enriched populations of optic vesicle-like retinal cells from human pluripotent stem cells
Abstract
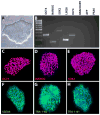
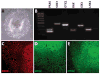
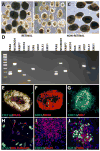
The protocol outlined below is used to differentiate human pluripotent stem cells (hPSCs) into retinal cell types through a process that faithfully recapitulates the stepwise progression observed in vivo. From pluripotency, cells are differentiated to a primitive anterior neural fate, followed by progression into two distinct populations of retinal progenitors and forebrain progenitors, each of which can be manually separated and purified. The hPSC-derived retinal progenitors are found to self-organize into three-dimensional optic vesicle-like structures, with each aggregate possessing the ability to differentiate into all major retinal cell types. The ability to faithfully recapitulate the stepwise in vivo development in a three-dimensional cell culture system allows for the study of mechanisms underlying human retinogenesis. Furthermore, this methodology allows for the study of retinal dysfunction and disease modeling using patient-derived cells, as well as high-throughput pharmacological screening and eventually patient-specific therapies.
Keywords: development; differentiation; human pluripotent stem cells (hPSCs); optic vesicle; retina.
Copyright © 2015 John Wiley & Sons, Inc.
Figures



Similar articles
-
New medium used in the differentiation of human pluripotent stem cells to retinal cells is comparable to fetal human eye tissue.Biomaterials. 2015 Jun;53:40-9. doi: 10.1016/j.biomaterials.2015.02.065. Epub 2015 Mar 9. Biomaterials. 2015. PMID: 25890705
-
Stem cells from apical papilla promote differentiation of human pluripotent stem cells towards retinal cells.Differentiation. 2018 May-Jun;101:8-15. doi: 10.1016/j.diff.2018.02.003. Epub 2018 Mar 2. Differentiation. 2018. PMID: 29574166
-
Differentiation of retinal organoids from human pluripotent stem cells.Methods Cell Biol. 2020;159:279-302. doi: 10.1016/bs.mcb.2020.02.005. Epub 2020 Mar 11. Methods Cell Biol. 2020. PMID: 32586447
-
Advances in the Differentiation of Retinal Ganglion Cells from Human Pluripotent Stem Cells.Adv Exp Med Biol. 2019;1186:121-140. doi: 10.1007/978-3-030-28471-8_5. Adv Exp Med Biol. 2019. PMID: 31654388 Free PMC article. Review.
-
Mimicking Retinal Development and Disease With Human Pluripotent Stem Cells.Invest Ophthalmol Vis Sci. 2016 Apr 1;57(5):ORSFf1-9. doi: 10.1167/iovs.15-18160. Invest Ophthalmol Vis Sci. 2016. PMID: 27116663 Review.
Cited by
-
Evaluation of photoreceptor-directed fibroblasts derived from retinitis pigmentosa patients with defects in the EYS gene: a possible cost-effective cellular model for mechanism-oriented drug.Stem Cell Res Ther. 2022 Apr 11;13(1):157. doi: 10.1186/s13287-022-02827-x. Stem Cell Res Ther. 2022. PMID: 35410372 Free PMC article.
-
Astrocytes modulate neurodegenerative phenotypes associated with glaucoma in OPTN(E50K) human stem cell-derived retinal ganglion cells.Stem Cell Reports. 2022 Jul 12;17(7):1636-1649. doi: 10.1016/j.stemcr.2022.05.006. Epub 2022 Jun 16. Stem Cell Reports. 2022. PMID: 35714595 Free PMC article.
-
Report on the National Eye Institute Audacious Goals Initiative: Photoreceptor Regeneration and Integration Workshop.Transl Vis Sci Technol. 2015 Nov 17;4(6):2. doi: 10.1167/tvst.4.6.2. eCollection 2015 Nov. Transl Vis Sci Technol. 2015. PMID: 26629398 Free PMC article.
-
Extension of retinofugal projections in an assembled model of human pluripotent stem cell-derived organoids.Stem Cell Reports. 2021 Sep 14;16(9):2228-2241. doi: 10.1016/j.stemcr.2021.05.009. Epub 2021 Jun 10. Stem Cell Reports. 2021. PMID: 34115986 Free PMC article.
-
Retina organoids: Window into the biophysics of neuronal systems.Biophys Rev (Melville). 2022 Jan 18;3(1):011302. doi: 10.1063/5.0077014. eCollection 2022 Mar. Biophys Rev (Melville). 2022. PMID: 38505227 Free PMC article. Review.
References
-
- Belecky-Adams T, Tomarev S, Li HS, Ploder L, McInnes RR, Sundin O, Adler R. Pax-6, Prox 1, and Chx10 homeobox gene expression correlates with phenotypic fate of retinal precursor cells. Invest Ophthalmol Vis Sci. 1997;38:1293–1303. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

