Contribution of liver alcohol dehydrogenase to metabolism of alcohols in rats
- PMID: 25641189
- PMCID: PMC4414724
- DOI: 10.1016/j.cbi.2014.12.040
Contribution of liver alcohol dehydrogenase to metabolism of alcohols in rats
Abstract
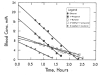
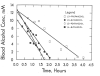
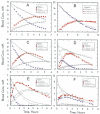
The kinetics of oxidation of various alcohols by purified rat liver alcohol dehydrogenase (ADH) were compared with the kinetics of elimination of the alcohols in rats in order to investigate the roles of ADH and other factors that contribute to the rates of metabolism of alcohols. Primary alcohols (ethanol, 1-propanol, 1-butanol, 2-methyl-1-propanol, 3-methyl-1-butanol) and diols (1,3-propanediol, 1,3-butanediol, 1,4-butanediol, 1,5-pentanediol) were eliminated in rats with zero-order kinetics at doses of 5-20 mmol/kg. Ethanol was eliminated most rapidly, at 7.9 mmol/kgh. Secondary alcohols (2-propanol-d7, 2-propanol, 2-butanol, 3-pentanol, cyclopentanol, cyclohexanol) were eliminated with first order kinetics at doses of 5-10 mmol/kg, and the corresponding ketones were formed and slowly eliminated with zero or first order kinetics. The rates of elimination of various alcohols were inhibited on average 73% (55% for 2-propanol to 90% for ethanol) by 1 mmol/kg of 4-methylpyrazole, a good inhibitor of ADH, indicating a major role for ADH in the metabolism of the alcohols. The Michaelis kinetic constants from in vitro studies (pH 7.3, 37 °C) with isolated rat liver enzyme were used to calculate the expected relative rates of metabolism in rats. The rates of elimination generally increased with increased activity of ADH, but a maximum rate of 6±1 mmol/kg h was observed for the best substrates, suggesting that ADH activity is not solely rate-limiting. Because secondary alcohols only require one NAD(+) for the conversion to ketones whereas primary alcohols require two equivalents of NAD(+) for oxidation to the carboxylic acids, it appears that the rate of oxidation of NADH to NAD(+) is not a major limiting factor for metabolism of these alcohols, but the rate-limiting factors are yet to be identified.
Keywords: Alcohol dehydrogenase; Alcohol metabolism; Enzyme specificity; Inhibition; Rat metabolism.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures

Similar articles
-
Cutaneous metabolism of glycol ethers.Arch Toxicol. 2005 Mar;79(3):160-8. doi: 10.1007/s00204-004-0619-3. Epub 2004 Nov 17. Arch Toxicol. 2005. PMID: 15551062
-
Comparison of the metabolism of alcohols by rat hepatic and pulmonary alcohol dehydrogenase.Biochem Mol Biol Int. 1995 Sep;37(1):65-71. Biochem Mol Biol Int. 1995. PMID: 8653089
-
Alteration in substrate specificity of horse liver alcohol dehydrogenase by an acyclic nicotinamide analog of NAD(+).DNA Repair (Amst). 2014 Nov;23:95-100. doi: 10.1016/j.dnarep.2014.09.005. Epub 2014 Oct 3. DNA Repair (Amst). 2014. PMID: 25280628
-
Use of the anti-Prelog stereospecific alcohol dehydrogenase from Leifsonia and Pseudomonas for producing chiral alcohols.Appl Microbiol Biotechnol. 2014 May;98(9):3889-904. doi: 10.1007/s00253-014-5619-5. Epub 2014 Mar 11. Appl Microbiol Biotechnol. 2014. PMID: 24615386 Review.
-
Mammalian liver alcohol dehydrogenases.Adv Exp Med Biol. 1975;56:1-31. doi: 10.1007/978-1-4684-7529-6_1. Adv Exp Med Biol. 1975. PMID: 167554 Review.
Cited by
-
Evaluation of intoxicating effects of liquor products on drunken mice.Medchemcomm. 2016 Oct 5;8(1):122-129. doi: 10.1039/c6md00491a. eCollection 2017 Jan 1. Medchemcomm. 2016. PMID: 30108697 Free PMC article.
-
Inversion of substrate stereoselectivity of horse liver alcohol dehydrogenase by substitutions of Ser-48 and Phe-93.Chem Biol Interact. 2017 Oct 1;276:77-87. doi: 10.1016/j.cbi.2016.12.016. Epub 2016 Dec 23. Chem Biol Interact. 2017. PMID: 28025168 Free PMC article.
-
Comparative 1H NMR-Based Chemometric Evaluations of the Time-Dependent Generation of Aldehydic Lipid Oxidation Products in Culinary Oils Exposed to Laboratory-Simulated Shallow Frying Episodes: Differential Patterns Observed for Omega-3 Fatty Acid-Containing Soybean Oils.Foods. 2021 Oct 17;10(10):2481. doi: 10.3390/foods10102481. Foods. 2021. PMID: 34681530 Free PMC article.
-
Does Oxidative Stress Induced by Alcohol Consumption Affect Orthodontic Treatment Outcome?Front Physiol. 2017 Jan 25;8:22. doi: 10.3389/fphys.2017.00022. eCollection 2017. Front Physiol. 2017. PMID: 28179886 Free PMC article. Review.
-
Characterizing the Hepatic Metabolic Pathway of Ketone Ester and Subsequent Metabolites Using Human and Rat Liver Fractions.AAPS J. 2025 Mar 14;27(2):65. doi: 10.1208/s12248-025-01044-7. AAPS J. 2025. PMID: 40087222
References
-
- Jörnvall H, Hedlund J, Bergman T, Kallberg Y, Cederlund E, Persson B. Origin and evolution of medium chain alcohol dehydrogenases. Chem. Biol. Interact. 2013;202:91–96. - PubMed
-
- Plapp BV. Rate-limiting steps in ethanol metabolism and approaches to changing these rates biochemically. In: Majchrowicz E, editor. Biochemical Pharmacology of Ethanol. Vol. 56. Plenum; New York: 1975. pp. 77–109. Adv. Exp. Med. Biol. - PubMed
-
- Havre P, Abrams MA, Corrall RJM, Yu LC, Szczepanik PA, Feldman HB, Klein P, Kong MS, Margolis JM, Landau BR. Quantitation of pathways of ethanol metabolism. Arch. Biochem. Biophys. 1977;182:14–23. - PubMed
-
- Cornell NW, Crow KE, Leadbetter MG, Veech RL, Li T-K, Schenker S, Lumeng L. Alcohol and Nutrition. U.S. Government Printing Office; Washington,D.C.: 1979. Rate determining factors for ethanol oxidation in rats in vivo and in isolated rat hepatocytes; pp. 315–330.
-
- Chen WS, Plapp BV. Kinetics and control of alcohol oxidation in rats. Adv. Exp. Med. Biol. 1980;132:543–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

