Discovery of a series of aromatic lactones as ALDH1/2-directed inhibitors
- PMID: 25641190
- PMCID: PMC4414788
- DOI: 10.1016/j.cbi.2014.12.038
Discovery of a series of aromatic lactones as ALDH1/2-directed inhibitors
Abstract
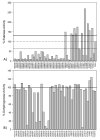
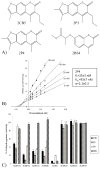
In humans, the aldehyde dehydrogenase superfamily consists of 19 isoenzymes which mostly catalyze the NAD(P)(+)-dependent oxidation of aldehydes. Many of these isoenzymes have overlapping substrate specificities and therefore their potential physiological functions may overlap. Thus the development of new isoenzyme-selective probes would be able to better delineate the function of a single isoenzyme and its individual contribution to the metabolism of a particular substrate. This specific study was designed to find a novel modulator of ALDH2, a mitochondrial ALDH isoenzyme most well-known for its role in acetaldehyde oxidation. 53 compounds were initially identified to modulate the activity of ALDH2 by a high-throughput esterase screen from a library of 63,000 compounds. Of these initial 53 compounds, 12 were found to also modulate the oxidation of propionaldehyde by ALDH2. Single concentration measurements at 10μM compound were performed using ALDH1A1, ALDH1A2, ALDH1A3, ALDH2, ALDH1B1, ALDH3A1, ALDH4A1, and/or ALDH5A1 to determine the selectivity of these 12 compounds toward ALDH2. Four of the twelve compounds shared an aromatic lactone structure and were found to be potent inhibitors of the ALDH1/2 isoenzymes, but have no inhibitory effect on ALDH3A1, ALDH4A1 or ALDH5A1. Two of the aromatic lactones show selectivity within the ALDH1/2 class, and one appears to be selective for ALDH2 compared to all other isoenzymes tested.
Keywords: Aldehyde dehydrogenase; High-throughput screening; Inhibition.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Thomas D. Hurley holds significant financial equity in SAJE Pharma, LLC. However, none of the work described in this study is related to, based on or supported by the company.
Figures
Similar articles
-
Inhibition of the Aldehyde Dehydrogenase 1/2 Family by Psoralen and Coumarin Derivatives.J Med Chem. 2017 Mar 23;60(6):2439-2455. doi: 10.1021/acs.jmedchem.6b01825. Epub 2017 Mar 6. J Med Chem. 2017. PMID: 28219011 Free PMC article.
-
Uncompetitive inhibition of Xenopus laevis aldehyde dehydrogenase 1A1 by divalent cations.Zoolog Sci. 2006 Mar;23(3):239-44. doi: 10.2108/zsj.23.239. Zoolog Sci. 2006. PMID: 16603817
-
Development of a high-throughput in vitro assay to identify selective inhibitors for human ALDH1A1.Chem Biol Interact. 2015 Jun 5;234:29-37. doi: 10.1016/j.cbi.2014.10.028. Epub 2014 Nov 4. Chem Biol Interact. 2015. PMID: 25450233 Free PMC article.
-
ALDH Enzymes and Hematological Diseases: A Scoping Review of Literature.Discov Med. 2024 Dec;36(191):2313-2324. doi: 10.24976/Discov.Med.202436191.213. Discov Med. 2024. PMID: 39726306
-
Aldehyde dehydrogenases and cell proliferation.Free Radic Biol Med. 2012 Feb 15;52(4):735-46. doi: 10.1016/j.freeradbiomed.2011.11.033. Epub 2011 Dec 21. Free Radic Biol Med. 2012. PMID: 22206977 Review.
Cited by
-
Inhibition of the Aldehyde Dehydrogenase 1/2 Family by Psoralen and Coumarin Derivatives.J Med Chem. 2017 Mar 23;60(6):2439-2455. doi: 10.1021/acs.jmedchem.6b01825. Epub 2017 Mar 6. J Med Chem. 2017. PMID: 28219011 Free PMC article.
-
Update of ALDH as a Potential Biomarker and Therapeutic Target for AML.Biomed Res Int. 2018 Jan 3;2018:9192104. doi: 10.1155/2018/9192104. eCollection 2018. Biomed Res Int. 2018. PMID: 29516013 Free PMC article. Review.
-
Bone marrow stromal cells induce an ALDH+ stem cell-like phenotype and enhance therapy resistance in AML through a TGF-β-p38-ALDH2 pathway.PLoS One. 2020 Nov 30;15(11):e0242809. doi: 10.1371/journal.pone.0242809. eCollection 2020. PLoS One. 2020. PMID: 33253299 Free PMC article.
-
The pathogenic role of retinoid nuclear receptor signaling in cancer and metabolic syndromes.J Exp Med. 2024 Sep 2;221(9):e20240519. doi: 10.1084/jem.20240519. Epub 2024 Aug 12. J Exp Med. 2024. PMID: 39133222 Free PMC article. Review.
References
-
- Vasiliou V, Pappa A, Estey T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug metabolism reviews. 2004;36:279–299. - PubMed
-
- Vasiliou V, Pappa A, Petersen DR. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chemico-biological interactions. 2000;129:1–19. - PubMed
-
- O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Critical reviews in toxicology. 2005;35:609–662. - PubMed
-
- Wong RH, Wang JD, Hsieh LL, Du CL, Cheng TJ. Effects on sister chromatid exchange frequency of aldehyde dehydrogenase 2 genotype and smoking in vinyl chloride workers. Mutation research. 1998;420:99–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

