New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3)
- PMID: 25641260
- PMCID: PMC4409854
- DOI: 10.1111/dom.12438
New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3)
Abstract
Aims: To compare the efficacy and safety of new insulin glargine 300 U/ml (Gla-300) with that of glargine 100 U/ml (Gla-100) in insulin-naïve people with type 2 diabetes using oral glucose-lowering drugs.
Methods: The EDITION 3 study was a multicentre, open-label, parallel-group study. Participants were randomized to Gla-300 or Gla-100 once daily for 6 months, discontinuing sulphonylureas and glinides, with a dose titration aimed at achieving pre-breakfast plasma glucose concentrations of 4.4-5.6 mmol/l (80-100 mg/dl). The primary endpoint was change in glycated haemoglobin (HbA1c) from baseline to month 6. The main secondary endpoint was percentage of participants with ≥1 nocturnal confirmed [≤3.9 mmol/l (≤70 mg/dl)] or severe hypoglycaemia from week 9 to month 6. Other measures of glycaemia and hypoglycaemia, weight change and insulin dose were assessed.
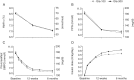
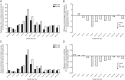
Results: Randomized participants (n = 878) had a mean (standard deviation) age of 57.7 (10.1) years, diabetes duration 9.8 (6.4) years, body mass index 33.0 (6.7) kg/m(2) and HbA1c 8.54 (1.06) % [69.8 (11.6) mmol/mol]. HbA1c levels decreased by equivalent amounts with the two treatments; the least squares mean difference in change from baseline was 0.04 [95% confidence interval (CI) -0.09 to 0.17] % or 0.4 (-1.0 to 1.9) mmol/mol. Numerically fewer participants reported ≥1 nocturnal confirmed (≤3.9 mmol/l) or severe hypoglycaemia from week 9 to month 6 [relative risk (RR) 0.89 (95% CI 0.66 to 1.20)] with Gla-300 versus Gla-100; a significantly lower risk of hypoglycaemia with this definition was found over the 6-month treatment period [RR 0.76 (95% CI 0.59 to 0.99)]. No between-treatment differences in adverse events were identified.
Conclusions: Gla-300 is as effective as Gla-100 in reducing HbA1c in insulin-naïve people with type 2 diabetes, with lower hypoglycaemia risk.
Trial registration: ClinicalTrials.gov NCT01676220.
Keywords: basal insulin analogues; basal insulin initiation; type 2 diabetes.
© 2015 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures

References
-
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. et al. - PMC - PubMed
-
- Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28:2543–2545. - PubMed
-
- Kunt T, Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract. 2009;63:6–10. (Suppl. 164): - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

