Indomethacin-induced impairment of regional cerebrovascular reactivity: implications for respiratory control
- PMID: 25641262
- PMCID: PMC4358685
- DOI: 10.1113/jphysiol.2014.284521
Indomethacin-induced impairment of regional cerebrovascular reactivity: implications for respiratory control
Abstract
Cerebrovascular reactivity impacts CO₂-[H(+)] washout at the central chemoreceptors and hence has marked influence on the control of ventilation. To date, the integration of cerebral blood flow (CBF) and ventilation has been investigated exclusively with measures of anterior CBF, which has a differential reactivity from the vertebrobasilar system and perfuses the brainstem. We hypothesized that: (1) posterior versus anterior CBF would have a stronger relationship to central chemoreflex magnitude during hypercapnia, and (2) that higher posterior reactivity would lead to a greater hypoxic ventilatory decline (HVD). End-tidal forcing was used to induce steady-state hyperoxic (300 mmHg P ET ,O₂) hypercapnia (+3, +6 and +9 mmHg P ET ,CO₂) and isocapnic hypoxia (45 mmHg P ET ,O₂) before and following pharmacological blunting (indomethacin; INDO; 1.45 ± 0.17 mg kg(-1)) of resting CBF and reactivity. In 22 young healthy volunteers, ventilation, intra-cranial arterial blood velocities and extra-cranial blood flows were measured during these challenges. INDO-induced blunting of cerebrovascular flow responsiveness (CVR) to CO₂ was unrelated to variability in ventilatory sensitivity during hyperoxic hypercapnia. Further results in a sub-group of volunteers (n = 9) revealed that elevations of P ET,CO₂ via end-tidal forcing reduce arterial-jugular venous gradients, attenuating the effect of CBF on chemoreflex responses. During isocapnic hypoxia, vertebral artery CVR was related to the magnitude of HVD (R(2) = 0.27; P < 0.04; n = 16), suggesting that CO₂-[H(+)] washout from central chemoreceptors modulates hypoxic ventilatory dynamics. No relationships were apparent with anterior CVR. As higher posterior, but not anterior, CVR was linked to HVD, our study highlights the importance of measuring flow in posterior vessels to investigate CBF and ventilatory integration.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures

 ). Dotted lines separate transition periods from steady-state end-tidal clamping. Test 2, isocapnic hypoxia had a 1 min baseline stage followed by 10 min of steady-state isocapnic hypoxia (+1
). Dotted lines separate transition periods from steady-state end-tidal clamping. Test 2, isocapnic hypoxia had a 1 min baseline stage followed by 10 min of steady-state isocapnic hypoxia (+1  , 45 Torr
, 45 Torr  ). HVR, HVD, and time to peak
). HVR, HVD, and time to peak  were calculated as shown in this figure. Subjects were then orally administered INDO, and the tests were repeated following 90 min.
were calculated as shown in this figure. Subjects were then orally administered INDO, and the tests were repeated following 90 min.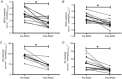
 ). A, absolute MCAv responses pre- and post INDO (cm s–1 (mmHg
). A, absolute MCAv responses pre- and post INDO (cm s–1 (mmHg  )−1; n = 13); B, absolute PCAv responses pre- and post INDO (cm s–1 (mmHg
)−1; n = 13); B, absolute PCAv responses pre- and post INDO (cm s–1 (mmHg  )−1); C, absolute ICA responses pre- and post INDO (ml min–1 (mmHg
)−1); C, absolute ICA responses pre- and post INDO (ml min–1 (mmHg  )−1); D, absolute VA responses pre- and post INDO (ml min–1 (mmHg
)−1); D, absolute VA responses pre- and post INDO (ml min–1 (mmHg  )−1). *Significant change from baseline post INDO administration (P < 0.05).
)−1). *Significant change from baseline post INDO administration (P < 0.05).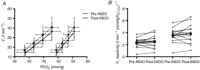
 ). Mean central chemoreflex responsiveness to elevations in both
). Mean central chemoreflex responsiveness to elevations in both  (▪ and □) and
(▪ and □) and  (• and ○). Error bars represent one standard deviation. B, individual (□ and ○) and mean (▪ and •) ventilatory reactivity to elevations in
(• and ○). Error bars represent one standard deviation. B, individual (□ and ○) and mean (▪ and •) ventilatory reactivity to elevations in  (▪ and □) and
(▪ and □) and  (• and ○).
(• and ○).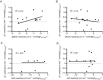
 sensitivity, within-subject (n = 13). B, ΔPCAv CO2 reactivity plotted against Δ
sensitivity, within-subject (n = 13). B, ΔPCAv CO2 reactivity plotted against Δ sensitivity, within-subject (n = 14). C, ΔICA CO2 reactivity plotted against ΔCCR reactivity, within-subject (n = 7). D, ΔVA CO2 reactivity plotted against Δ
sensitivity, within-subject (n = 14). C, ΔICA CO2 reactivity plotted against ΔCCR reactivity, within-subject (n = 7). D, ΔVA CO2 reactivity plotted against Δ sensitivity, within-subject (n = 12).
sensitivity, within-subject (n = 12).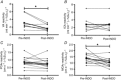
 )−1; n = 7); B, absolute ICA responses pre- and post INDO (ml min–1 (–%
)−1; n = 7); B, absolute ICA responses pre- and post INDO (ml min–1 (–% )−1; n = 7). C, absolute PCAv responses pre- and post INDO (cm s–1 (–%
)−1; n = 7). C, absolute PCAv responses pre- and post INDO (cm s–1 (–% )−1; n = 14); D, absolute MCAv responses pre- and post INDO (cm s–1 (–%
)−1; n = 14); D, absolute MCAv responses pre- and post INDO (cm s–1 (–% )−1; n = 13); *Significant change from baseline post INDO administration (P < 0.05).
)−1; n = 13); *Significant change from baseline post INDO administration (P < 0.05).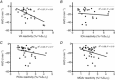

 (or
(or  ), as is seen in our placebo/INDO intervention. The major limitation of this model is that if we use C2 to represent arterial blood vessels proximal to the central chemoreceptors, and C1 to represent the tissue compartment where the central chemoreceptors reside, C1 is, in contrast to a steady-state, constantly producing CO2 via metabolism. Therefore, a simple square wave change cannot be assumed between blood and tissue CO2, but for simplicity we can assume that the relationship between arterial and tissue CO2 resides somewhere on the dashed line, based upon the magnitude of flow and metabolic production of CO2 at any given time. Assuming constant CO2 production, higher flow would result in a rightward shift down the line, and an overall reduced blood to tissue gradient.
), as is seen in our placebo/INDO intervention. The major limitation of this model is that if we use C2 to represent arterial blood vessels proximal to the central chemoreceptors, and C1 to represent the tissue compartment where the central chemoreceptors reside, C1 is, in contrast to a steady-state, constantly producing CO2 via metabolism. Therefore, a simple square wave change cannot be assumed between blood and tissue CO2, but for simplicity we can assume that the relationship between arterial and tissue CO2 resides somewhere on the dashed line, based upon the magnitude of flow and metabolic production of CO2 at any given time. Assuming constant CO2 production, higher flow would result in a rightward shift down the line, and an overall reduced blood to tissue gradient.
 pre- and post INDO (continuous and dotted lines, respectively) during hypercapnia as a function of steady-state fractional inspired CO2 (Xie et al. 2006), rebreathing (Fan et al. 2010a), and end-tidal forcing methods of controlling
pre- and post INDO (continuous and dotted lines, respectively) during hypercapnia as a function of steady-state fractional inspired CO2 (Xie et al. 2006), rebreathing (Fan et al. 2010a), and end-tidal forcing methods of controlling  (current study). The bottom panel represents the changes in
(current study). The bottom panel represents the changes in  −
−  gradient expected using each method. A, for the steady-state method, the pre-INDO gradient data points (•) are calculated from Peebles et al. , while the post-INDO data points (○) are theoretical. As INDO-induced reductions in cerebrovascular CO2 reactivity should reduce CO2 washout, it is expected that
gradient expected using each method. A, for the steady-state method, the pre-INDO gradient data points (•) are calculated from Peebles et al. , while the post-INDO data points (○) are theoretical. As INDO-induced reductions in cerebrovascular CO2 reactivity should reduce CO2 washout, it is expected that  is not reduced to as great an extent post INDO, keeping the
is not reduced to as great an extent post INDO, keeping the  −
−  gradient larger than in the pre-INDO scenario and resulting in increased ventilatory sensitivity. B, during rebreathing, the gradient between
gradient larger than in the pre-INDO scenario and resulting in increased ventilatory sensitivity. B, during rebreathing, the gradient between  and
and  is theoretically eliminated (Read & Leigh, 1967) and is independent of reactivity and thus should be the same pre- (•) and post INDO (○). This latter notion is supported by no changes in ventilatory sensitivity pre- and post INDO using the rebreathing method (Fan et al. 2010a). C, similar to the rebreathing method, we found no change in ventilatory sensitivity pre- and post INDO with end-tidal forcing. The pre-INDO (•) data points represent the
is theoretically eliminated (Read & Leigh, 1967) and is independent of reactivity and thus should be the same pre- (•) and post INDO (○). This latter notion is supported by no changes in ventilatory sensitivity pre- and post INDO using the rebreathing method (Fan et al. 2010a). C, similar to the rebreathing method, we found no change in ventilatory sensitivity pre- and post INDO with end-tidal forcing. The pre-INDO (•) data points represent the  −
−  gradient recorded from previously collected data in our laboratory, while post-INDO (○) points represent the likelihood that
gradient recorded from previously collected data in our laboratory, while post-INDO (○) points represent the likelihood that  −
−  gradient magnitude was unchanged, explaining the similar ventilatory sensitivity pre- and post INDO.
gradient magnitude was unchanged, explaining the similar ventilatory sensitivity pre- and post INDO.References
-
- Ainslie PN. Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473–R1495. - PubMed
-
- Ainslie PN. Hoiland RL. Transcranial Doppler ultrasound: valid, invalid, or both? J Appl Physiol. 2014;117:1081–1083. - PubMed
-
- Ainslie PN, Shaw AD, Smith KJ, Willie CK, Ikeda K, Graham J. Macleod DB. Stability of cerebral metabolism and substrate availability in humans during hypoxia and hyperoxia. Clin Sci (Lond) 2014;126:661–670. - PubMed
-
- Black MA, Cable NT, Thijssen DHJ. Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension. 2008;51:203–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

