Exploiting algal NADPH oxidase for biophotovoltaic energy
- PMID: 25641364
- PMCID: PMC5016757
- DOI: 10.1111/pbi.12332
Exploiting algal NADPH oxidase for biophotovoltaic energy
Abstract
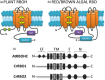
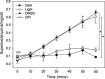
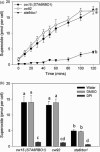
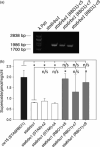
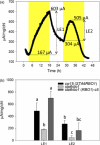
Photosynthetic microbes exhibit light-dependent electron export across the cell membrane, which can generate electricity in biological photovoltaic (BPV) devices. How electrons are exported remains to be determined; the identification of mechanisms would help selection or generation of photosynthetic microbes capable of enhanced electrical output. We show that plasma membrane NADPH oxidase activity is a significant component of light-dependent generation of electricity by the unicellular green alga Chlamydomonas reinhardtii. NADPH oxidases export electrons across the plasma membrane to form superoxide anion from oxygen. The C. reinhardtii mutant lacking the NADPH oxidase encoded by RBO1 is impaired in both extracellular superoxide anion production and current generation in a BPV device. Complementation with the wild-type gene restores both capacities, demonstrating the role of the enzyme in electron export. Monitoring light-dependent extracellular superoxide production with a colorimetric assay is shown to be an effective way of screening for electrogenic potential of candidate algal strains. The results show that algal NADPH oxidases are important for superoxide anion production and open avenues for optimizing the biological component of these devices.
Keywords: Chlamydomonas; NADPH oxidase; alga; biophotovoltaic; energy.
© 2015 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures

References
-
- Allen, M.D. , del Campo, J.A. , Kropat, J. and Merchant, S.S. (2007) FEA1, FEA2, and FRE1, encoding two homologous secreted proteins and a candidate ferrireductase, are expressed coordinately with FOX1 and FTR1 in iron‐deficient Chlamydomonas reinhardtii . Eukaryot. Cell 6, 1841–1852. - PMC - PubMed
-
- Anderson, A. , Bothwell, J.H. , Laohavisit, A. , Smith, A.G. and Davies, J.M. (2011) NOX or not? Evidence for algal NADPH oxidases. Trends Plant Sci. 16, 579–581. - PubMed
-
- Blaby, I.K. , Glaesner, A.G. , Mettler, T. , Fitz‐Gibbon, S.T. , Gallaher, S.D. , Liu, B.S. , Boyle, N.R. , Kropat, J. , Stitt, M. , Johnson, S. , Benning, C. , Pellegrini, M. , Casero, D. and Merchant, S.S. (2013) Systems‐level analysis of nitrogen starvation‐induced modifications of carbon metabolism in a Chlamydomonas reinhardtii starchless mutant. Plant Cell, 25, 4305–4323. - PMC - PubMed
-
- Bombelli, P. , Bradley, R.W. , Scott, A.M. , Philips, A.J. , McCormick, A.J. , Cruz, S.M. , Anderson, A.A. , Yunus, K. , Bendall, D.S. , Cameron, P. , Davies, J.M. , Smith, A.G. , Howe, C.J. and Fisher, A.C. (2011) Quantitative analysis of the factors limiting solar power transduction by Synechocystis sp. PCC 6803 in biological photovoltaic devices. Energy Environ. Sci. 4, 4690–4698.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

