Genetic modifiers of ambulation in the Cooperative International Neuromuscular Research Group Duchenne Natural History Study
- PMID: 25641372
- PMCID: PMC4403971
- DOI: 10.1002/ana.24370
Genetic modifiers of ambulation in the Cooperative International Neuromuscular Research Group Duchenne Natural History Study
Abstract
Objective: We studied the effects of LTBP4 and SPP1 polymorphisms on age at loss of ambulation (LoA) in a multiethnic Duchenne muscular dystrophy (DMD) cohort.
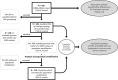
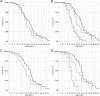
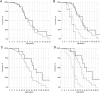
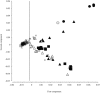
Methods: We genotyped SPP1 rs28357094 and LTBP4 haplotype in 283 of 340 participants in the Cooperative International Neuromuscular Research Group Duchenne Natural History Study (CINRG-DNHS). Median ages at LoA were compared by Kaplan-Meier analysis and log-rank test. We controlled polymorphism analyses for concurrent effects of glucocorticoid corticosteroid (GC) treatment (time-varying Cox regression) and for population stratification (multidimensional scaling of genome-wide markers).
Results: Hispanic and South Asian participants (n = 18, 41) lost ambulation 2.7 and 2 years earlier than Caucasian subjects (p = 0.003, <0.001). The TG/GG genotype at SPP1 rs28357094 was associated to 1.2-year-earlier median LoA (p = 0.048). This difference was greater (1.9 years, p = 0.038) in GC-treated participants, whereas no difference was observed in untreated subjects. Cox regression confirmed a significant effect of SPP1 genotype in GC-treated participants (hazard ratio = 1.61, p = 0.016). LTBP4 genotype showed a direction of association with age at LoA as previously reported, but it was not statistically significant. After controlling for population stratification, we confirmed a strong effect of LTBP4 genotype in Caucasians (2.4 years, p = 0.024). Median age at LoA with the protective LTBP4 genotype in this cohort was 15.0 years, 16.0 for those who were treated with GC.
Interpretation: SPP1 rs28357094 acts as a pharmacodynamic biomarker of GC response, and LTBP4 haplotype modifies age at LoA in the CINRG-DNHS cohort. Adjustment for GC treatment and population stratification appears crucial in assessing genetic modifiers in DMD.
© 2015 The Authors Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures
Similar articles
-
LTBP4, SPP1, and CD40 Variants: Genetic Modifiers of Duchenne Muscular Dystrophy Analyzed in Serbian Patients.Genes (Basel). 2022 Aug 4;13(8):1385. doi: 10.3390/genes13081385. Genes (Basel). 2022. PMID: 36011296 Free PMC article.
-
Validation of genetic modifiers for Duchenne muscular dystrophy: a multicentre study assessing SPP1 and LTBP4 variants.J Neurol Neurosurg Psychiatry. 2015 Oct;86(10):1060-5. doi: 10.1136/jnnp-2014-308409. Epub 2014 Dec 4. J Neurol Neurosurg Psychiatry. 2015. PMID: 25476005 Free PMC article.
-
Genetic Modifiers of Duchenne Muscular Dystrophy and Dilated Cardiomyopathy.PLoS One. 2015 Oct 29;10(10):e0141240. doi: 10.1371/journal.pone.0141240. eCollection 2015. PLoS One. 2015. PMID: 26513582 Free PMC article.
-
Genetic Modifiers and Phenotype of Duchenne Muscular Dystrophy: A Systematic Review and Meta-Analysis.Pharmaceuticals (Basel). 2021 Aug 13;14(8):798. doi: 10.3390/ph14080798. Pharmaceuticals (Basel). 2021. PMID: 34451895 Free PMC article. Review.
-
Modifier genes and their effect on Duchenne muscular dystrophy.Curr Opin Neurol. 2015 Oct;28(5):528-34. doi: 10.1097/WCO.0000000000000240. Curr Opin Neurol. 2015. PMID: 26263473 Free PMC article. Review.
Cited by
-
DMD genotypes and loss of ambulation in the CINRG Duchenne Natural History Study.Neurology. 2016 Jul 26;87(4):401-9. doi: 10.1212/WNL.0000000000002891. Epub 2016 Jun 24. Neurology. 2016. PMID: 27343068 Free PMC article.
-
The skeletal muscle phenotype of the DE50-MD dog model of Duchenne muscular dystrophy.Wellcome Open Res. 2022 Sep 23;7:238. doi: 10.12688/wellcomeopenres.18251.1. eCollection 2022. Wellcome Open Res. 2022. PMID: 36865375 Free PMC article.
-
Mutation-Based Therapeutic Strategies for Duchenne Muscular Dystrophy: From Genetic Diagnosis to Therapy.J Pers Med. 2019 Mar 4;9(1):16. doi: 10.3390/jpm9010016. J Pers Med. 2019. PMID: 30836656 Free PMC article. Review.
-
Evidence for ACTN3 as a genetic modifier of Duchenne muscular dystrophy.Nat Commun. 2017 Jan 31;8:14143. doi: 10.1038/ncomms14143. Nat Commun. 2017. PMID: 28139640 Free PMC article.
-
Association of genetic mutations and loss of ambulation in childhood-onset dystrophinopathy.Muscle Nerve. 2021 Feb;63(2):181-191. doi: 10.1002/mus.27113. Epub 2020 Nov 17. Muscle Nerve. 2021. PMID: 33150975 Free PMC article.
References
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Bushby K, Finkel R, Birnkrant DJ. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. - PubMed
-
- Bushby K, Finkel R, Birnkrant DJ. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1RR031988/RR/NCRR NIH HHS/United States
- U54 RR026139/RR/NCRR NIH HHS/United States
- U54HD053177/HD/NICHD NIH HHS/United States
- R01AR062380/AR/NIAMS NIH HHS/United States
- R01 AR062380/AR/NIAMS NIH HHS/United States
- R24HD050846/HD/NICHD NIH HHS/United States
- UL1RR024992/RR/NCRR NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- G12RR003051/RR/NCRR NIH HHS/United States
- G12 RR003051/RR/NCRR NIH HHS/United States
- 1R01AR061875/AR/NIAMS NIH HHS/United States
- U54 HD053177/HD/NICHD NIH HHS/United States
- UL1 RR031988/RR/NCRR NIH HHS/United States
- U54RR026139/RR/NCRR NIH HHS/United States
- R24 HD050846/HD/NICHD NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- R01 AR061875/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

