Targeted Chemotherapy with Metal Complexes
- PMID: 25642012
- PMCID: PMC4309285
- DOI: 10.1080/02603594.2014.890099
Targeted Chemotherapy with Metal Complexes
Abstract
Classical chemotherapeutics, such as cisplatin and its analogues, have been highly successful in the clinic, yet improvements can certainly be made, given the significant side effects associated with the killing of healthy cells. Recent advances in the field of chemotherapy include the development of targeted anticancer agents, compounds that are directed towards a specific biomarker of cancer, with the hopes that such targeted therapies might have reduced side effects given their greater selectivity. Here we discuss several transition metal complexes that are tailored towards various biomolecules associated with cancer. Most notably, the success of rhodium metalloinsertors, which specifically bind to nucleic acid base mismatches in DNA, highlight the enormous potential of this exciting new strategy.
Keywords: DNA mismatch repair; bioinorganic; medicinal chemistry.
Figures

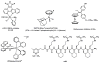


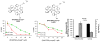
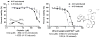
Similar articles
-
Targeting DNA Mismatches with Rhodium Metalloinsertors.Inorganica Chim Acta. 2016 Oct 1;452:3-11. doi: 10.1016/j.ica.2016.01.021. Epub 2016 Jan 16. Inorganica Chim Acta. 2016. PMID: 27746507 Free PMC article.
-
A Family of Rhodium Complexes with Selective Toxicity toward Mismatch Repair-Deficient Cancers.J Am Chem Soc. 2018 Apr 25;140(16):5612-5624. doi: 10.1021/jacs.8b02271. Epub 2018 Apr 17. J Am Chem Soc. 2018. PMID: 29620877 Free PMC article.
-
An unusual ligand coordination gives rise to a new family of rhodium metalloinsertors with improved selectivity and potency.J Am Chem Soc. 2014 Oct 8;136(40):14160-72. doi: 10.1021/ja5072064. Epub 2014 Sep 25. J Am Chem Soc. 2014. PMID: 25254630 Free PMC article.
-
DNA binding mode of ruthenium complexes and relationship to tumor cell toxicity.Drug Resist Updat. 2006 Jun;9(3):111-22. doi: 10.1016/j.drup.2006.05.002. Epub 2006 Jun 21. Drug Resist Updat. 2006. PMID: 16790363 Review.
-
Scope of organometallic compounds based on transition metal-arene systems as anticancer agents: starting from the classical paradigm to targeting multiple strategies.RSC Adv. 2019 Jan 24;9(6):3239-3278. doi: 10.1039/c8ra07926a. eCollection 2019 Jan 22. RSC Adv. 2019. PMID: 35518979 Free PMC article. Review.
Cited by
-
Targeting DNA Mismatches with Rhodium Metalloinsertors.Inorganica Chim Acta. 2016 Oct 1;452:3-11. doi: 10.1016/j.ica.2016.01.021. Epub 2016 Jan 16. Inorganica Chim Acta. 2016. PMID: 27746507 Free PMC article.
-
Peptide-Drug Conjugates and Their Targets in Advanced Cancer Therapies.Front Chem. 2020 Jul 7;8:571. doi: 10.3389/fchem.2020.00571. eCollection 2020. Front Chem. 2020. PMID: 32733853 Free PMC article. Review.
-
New Ru(II) complexes for dual photoreactivity: ligand exchange and (1)O2 generation.Acc Chem Res. 2015 Aug 18;48(8):2280-7. doi: 10.1021/acs.accounts.5b00227. Epub 2015 Jul 17. Acc Chem Res. 2015. PMID: 26186416 Free PMC article.
-
Rhodium metalloinsertor binding generates a lesion with selective cytotoxicity for mismatch repair-deficient cells.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):6948-6953. doi: 10.1073/pnas.1706665114. Epub 2017 Jun 20. Proc Natl Acad Sci U S A. 2017. PMID: 28634291 Free PMC article.
-
Clinical Risk Factors Influencing Dental Developmental Disturbances in Childhood Cancer Survivors.Cancer Res Treat. 2018 Jul;50(3):926-935. doi: 10.4143/crt.2017.296. Epub 2017 Oct 10. Cancer Res Treat. 2018. PMID: 29020731 Free PMC article.
References
-
- Mansour VH, Rosenberg B, Vancamp L, Trosko JE. Nature. 1969;222:385–386. - PubMed
-
- Wheate NJ, Walker S, Craig GE, Oun R. Dalton Trans. 2010;39:8113–8127. - PubMed
-
- Kelland LR, Sharp SY, O’Neill CF, Raynaud FI, Beale PJ, Judson IR. J Inorg Biochem. 1999;77:111–115. - PubMed
-
- Jamieson ER, Lippard SJ. Chem Rev. 1999;99:2467–2498. - PubMed
-
- Wang D, Lippard SJ. Nat Rev Drug Discovery. 2005;4:307–320. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
