Glial metabotropic glutamate receptor-4 increases maturation and survival of oligodendrocytes
- PMID: 25642169
- PMCID: PMC4294134
- DOI: 10.3389/fncel.2014.00462
Glial metabotropic glutamate receptor-4 increases maturation and survival of oligodendrocytes
Abstract
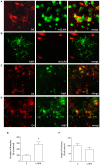
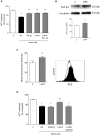
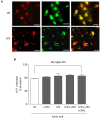
Group III metabotropic glutamate (mGlu) receptors mediate important neuroprotective and anti-inflammatory effects. Stimulation of mGlu4 receptor reduces neuroinflammation in a mouse model of experimental autoimmune encephalomyelitis (EAE) whereas mGlu4 knockout mice display exacerbated EAE clinical scores. We now show that mGlu4 receptors are expressed in oligodendrocytes, astrocytes and microglia in culture. Oligodendrocytes express mGlu4 receptors only at early stages of maturation (O4 positive), but not when more differentiated (myelin basic protein, MBP positive). Treatment of immature oligodendrocytes with the mGlu4 receptor agonist L-2-Amino-4-phosphonobutyrate (L-AP4; 50 μM for 48 h) accelerates differentiation with enhanced branching and earlier appearance of MBP staining. Oligodendrocyte death induced by exposure to 1 mM kainic acid for 24 h is significantly reduced by a 30-min pretreatment with L-AP4 (50 μM), an effect observed only in the presence of astrocytes, mimicked by the specific mGlu4 receptor positive allosteric modulator N-Phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC) (30 μM) and prevented by pretreatment with the mGlu4 receptor antagonist, cyclopropyl-4-phosphonophenylglycine (CPPG) (100 μM). In astrocytes, mGlu4 receptor is the most expressed among group III mGlu receptors, as by Quantitative real time PCR (QRT-PCR), and its silencing prevents protective effects. Protection is also observed when conditioned medium (CM) from L-AP4-pretreated astrocytes is transferred to oligodendrocytes challenged with kainic acid. Transforming growth factor β (TGF-β) mediates the increased oligodendrocyte survival as the effect of L-AP4 is mimicked by addition of 10 ng/ml TGF-β and prevented by incubation with a neutralizing anti-TGF-β antibody. In contrast, despite the expression of mGlu4 receptor in resting and activated microglia, CM from L-AP4-stimulated microglia does not modify kainate-induced oligodendrocyte toxicity. Our results suggest that mGlu4 receptors expressed in astrocytes mediate enhanced survival of oligodendrocytes under conditions of excitotoxicity.
Keywords: astrocytes; experimental autoimmune encephalomyelitis; multiple sclerosis; oligodendrocytes; transforming growth factor β.
Figures



Similar articles
-
Cinnabarinic acid, an endogenous agonist of type-4 metabotropic glutamate receptor, suppresses experimental autoimmune encephalomyelitis in mice.Neuropharmacology. 2014 Jun;81:237-43. doi: 10.1016/j.neuropharm.2014.02.011. Epub 2014 Feb 21. Neuropharmacology. 2014. PMID: 24565643
-
Combined administration of PHCCC, a positive allosteric modulator of mGlu4 receptors and ACPT-I, mGlu III receptor agonist evokes antidepressant-like effects in rats.Amino Acids. 2007 Feb;32(2):169-72. doi: 10.1007/s00726-006-0316-z. Epub 2006 Aug 2. Amino Acids. 2007. PMID: 16868652
-
Activation of group III metabotropic glutamate receptors inhibits the production of RANTES in glial cell cultures.J Neurosci. 2002 Jul 1;22(13):5403-11. doi: 10.1523/JNEUROSCI.22-13-05403.2002. J Neurosci. 2002. PMID: 12097492 Free PMC article.
-
Metabotropic Glutamate Receptors in Glial Cells: A New Potential Target for Neuroprotection?Front Mol Neurosci. 2018 Nov 13;11:414. doi: 10.3389/fnmol.2018.00414. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30483053 Free PMC article. Review.
-
Metabotropic glutamate receptors in neurodegeneration/neuroprotection: still a hot topic?Neurochem Int. 2012 Sep;61(4):559-65. doi: 10.1016/j.neuint.2012.01.017. Epub 2012 Jan 25. Neurochem Int. 2012. PMID: 22306345 Review.
Cited by
-
Impact of Neuron-Derived HGF on c-Met and KAI-1 in CNS Glial Cells: Implications for Multiple Sclerosis Pathology.Int J Mol Sci. 2024 Oct 19;25(20):11261. doi: 10.3390/ijms252011261. Int J Mol Sci. 2024. PMID: 39457044 Free PMC article.
-
Electrophysiological properties of NG2(+) cells: Matching physiological studies with gene expression profiles.Brain Res. 2016 May 1;1638(Pt B):138-160. doi: 10.1016/j.brainres.2015.09.010. Epub 2015 Sep 15. Brain Res. 2016. PMID: 26385417 Free PMC article. Review.
-
The Role of Neuroglial Metabotropic Glutamate Receptors in Alzheimer's Disease.Curr Neuropharmacol. 2023;21(2):273-283. doi: 10.2174/1570159X19666210916102638. Curr Neuropharmacol. 2023. PMID: 34530715 Free PMC article. Review.
-
G Protein-Coupled Receptors in Myelinating Glia.Trends Pharmacol Sci. 2016 Nov;37(11):977-987. doi: 10.1016/j.tips.2016.09.002. Epub 2016 Sep 23. Trends Pharmacol Sci. 2016. PMID: 27670389 Free PMC article. Review.
-
Systematic Review of Pharmacological Properties of the Oligodendrocyte Lineage.Front Cell Neurosci. 2016 Feb 12;10:27. doi: 10.3389/fncel.2016.00027. eCollection 2016. Front Cell Neurosci. 2016. PMID: 26903812 Free PMC article. Review.
References
-
- Blazevski J., Petkovic F., Momcilovic M., Jevtic B., Miljkovic D., Mostarica Stojkovic M. (2013). High interleukin-10 expression within the central nervous system may be important for initiation of recovery of Dark Agouti rats from experimental autoimmune encephalomyelitis. Immunobiology 218, 1192–1199. 10.1016/j.imbio.2013.04.004 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

