Marine sediments microbes capable of electrode oxidation as a surrogate for lithotrophic insoluble substrate metabolism
- PMID: 25642220
- PMCID: PMC4294203
- DOI: 10.3389/fmicb.2014.00784
Marine sediments microbes capable of electrode oxidation as a surrogate for lithotrophic insoluble substrate metabolism
Abstract
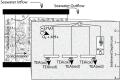
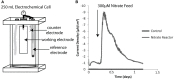
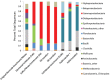
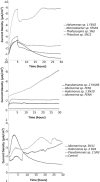
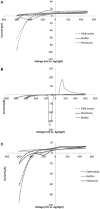
Little is known about the importance and/or mechanisms of biological mineral oxidation in sediments, partially due to the difficulties associated with culturing mineral-oxidizing microbes. We demonstrate that electrochemical enrichment is a feasible approach for isolation of microbes capable of gaining electrons from insoluble minerals. To this end we constructed sediment microcosms and incubated electrodes at various controlled redox potentials. Negative current production was observed in incubations and increased as redox potential decreased (tested -50 to -400 mV vs. Ag/AgCl). Electrode-associated biomass responded to the addition of nitrate and ferric iron as terminal electron acceptors in secondary sediment-free enrichments. Elemental sulfur, elemental iron and amorphous iron sulfide enrichments derived from electrode biomass demonstrated products indicative of sulfur or iron oxidation. The microbes isolated from these enrichments belong to the genera Halomonas, Idiomarina, Marinobacter, and Pseudomonas of the Gammaproteobacteria, and Thalassospira and Thioclava from the Alphaproteobacteria. Chronoamperometry data demonstrates sustained electrode oxidation from these isolates in the absence of alternate electron sources. Cyclic voltammetry demonstrated the variability in dominant electron transfer modes or interactions with electrodes (i.e., biofilm, planktonic or mediator facilitated) and the wide range of midpoint potentials observed for each microbe (from 8 to -295 mV vs. Ag/AgCl). The diversity of extracellular electron transfer mechanisms observed in one sediment and one redox condition, illustrates the potential importance and abundance of these interactions. This approach has promise for increasing our understanding the extent and diversity of microbe mineral interactions, as well as increasing the repository of microbes available for electrochemical applications.
Keywords: Halomonas; Marinobacter; Pseudomonas; electromicrobiology; geobiology; iron oxidation; lithotrophy; sulfur oxidation.
Figures

Similar articles
-
Differences in Applied Redox Potential on Cathodes Enrich for Diverse Electrochemically Active Microbial Isolates From a Marine Sediment.Front Microbiol. 2019 Aug 28;10:1979. doi: 10.3389/fmicb.2019.01979. eCollection 2019. Front Microbiol. 2019. PMID: 31555224 Free PMC article.
-
Variation in electrode redox potential selects for different microorganisms under cathodic current flow from electrodes in marine sediments.Environ Microbiol. 2018 Jun;20(6):2270-2287. doi: 10.1111/1462-2920.14275. Epub 2018 Aug 8. Environ Microbiol. 2018. PMID: 29786168
-
Cultivation of an obligate Fe(II)-oxidizing lithoautotrophic bacterium using electrodes.mBio. 2013 Jan 29;4(1):e00420-12. doi: 10.1128/mBio.00420-12. mBio. 2013. PMID: 23362318 Free PMC article.
-
[Oxidation of inorganic sulfur compounds by obligatory organotrophic bacteria].Mikrobiologiia. 2003 Nov-Dec;72(6):725-39. Mikrobiologiia. 2003. PMID: 14768537 Review. Russian.
-
Microbes, cables, and an electrical touch.Int Microbiol. 2015 Sep;18(3):151-7. doi: 10.2436/20.1501.01.245. Int Microbiol. 2015. PMID: 27036742 Review.
Cited by
-
NADH dehydrogenases drive inward electron transfer in Shewanella oneidensis MR-1.Microb Biotechnol. 2023 Mar;16(3):560-568. doi: 10.1111/1751-7915.14175. Epub 2022 Nov 24. Microb Biotechnol. 2023. PMID: 36420671 Free PMC article.
-
Genome-Scale Mutational Analysis of Cathode-Oxidizing Thioclava electrotropha ElOx9T.Front Microbiol. 2022 Jun 10;13:909824. doi: 10.3389/fmicb.2022.909824. eCollection 2022. Front Microbiol. 2022. PMID: 35756027 Free PMC article.
-
Syntrophic anaerobic photosynthesis via direct interspecies electron transfer.Nat Commun. 2017 Jan 9;8:13924. doi: 10.1038/ncomms13924. Nat Commun. 2017. PMID: 28067226 Free PMC article.
-
Relative abundance of 'Candidatus Tenderia electrophaga' is linked to cathodic current in an aerobic biocathode community.Microb Biotechnol. 2018 Jan;11(1):98-111. doi: 10.1111/1751-7915.12757. Epub 2017 Jul 11. Microb Biotechnol. 2018. PMID: 28696003 Free PMC article.
-
Bioelectricity (electromicrobiology) and sustainability.Microb Biotechnol. 2017 Sep;10(5):1114-1119. doi: 10.1111/1751-7915.12834. Epub 2017 Aug 14. Microb Biotechnol. 2017. PMID: 28805347 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

