Chemoautotrophic growth of ammonia-oxidizing Thaumarchaeota enriched from a pelagic redox gradient in the Baltic Sea
- PMID: 25642221
- PMCID: PMC4295551
- DOI: 10.3389/fmicb.2014.00786
Chemoautotrophic growth of ammonia-oxidizing Thaumarchaeota enriched from a pelagic redox gradient in the Baltic Sea
Abstract
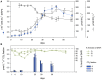
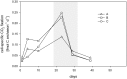
Ammonia-oxidizing archaea (AOA) are an important component of the planktonic community in aquatic habitats, linking nitrogen and carbon cycles through nitrification and carbon fixation. Therefore, measurements of these processes in culture-based experiments can provide insights into their contributions to energy conservation and biomass production by specific AOA. In this study, by enriching AOA from a brackish, oxygen-depleted water-column in the Landsort Deep, central Baltic Sea, we were able to investigate ammonium oxidation, chemoautotrophy, and growth in seawater batch experiments. The highly enriched culture consisted of up to 97% archaea, with maximal archaeal numbers of 2.9 × 10(7) cells mL(-1). Phylogenetic analysis of the 16S rRNA and ammonia monooxygenase subunit A (amoA) gene sequences revealed an affiliation with assemblages from low-salinity and freshwater habitats, with Candidatus Nitrosoarchaeum limnia as the closest relative. Growth correlated significantly with nitrite production, ammonium consumption, and CO2 fixation, which occurred at a ratio of 10 atoms N oxidized per 1 atom C fixed. According to the carbon balance, AOA biomass production can be entirely explained by chemoautotrophy. The cellular carbon content was estimated to be 9 fg C per cell. Single-cell-based (13)C and (15)N labeling experiments and analysis by nano-scale secondary ion mass spectrometry provided further evidence that cellular carbon was derived from bicarbonate and that ammonium was taken up by the cells. Our study therefore revealed that growth by an AOA belonging to the genus Nitrosoarchaeum can be sustained largely by chemoautotrophy.
Keywords: Baltic Sea; CO2-fixation; Thaumarchaeota; ammonia-oxidizing archaea; chemoautotrophy; enrichment.
Figures



Similar articles
-
Significance of archaeal nitrification in hypoxic waters of the Baltic Sea.ISME J. 2015 Jun;9(6):1319-32. doi: 10.1038/ismej.2014.218. Epub 2014 Nov 25. ISME J. 2015. PMID: 25423026 Free PMC article.
-
Nitrogen and Oxygen Isotope Effects of Ammonia Oxidation by Thermophilic Thaumarchaeota from a Geothermal Water Stream.Appl Environ Microbiol. 2016 Jul 15;82(15):4492-504. doi: 10.1128/AEM.00250-16. Print 2016 Aug 1. Appl Environ Microbiol. 2016. PMID: 27208107 Free PMC article.
-
Ecophysiology of an ammonia-oxidizing archaeon adapted to low-salinity habitats.Microb Ecol. 2012 Nov;64(4):955-63. doi: 10.1007/s00248-012-0075-1. Epub 2012 May 30. Microb Ecol. 2012. PMID: 22644483
-
Contribution of ammonia oxidation to chemoautotrophy in Antarctic coastal waters.ISME J. 2016 Nov;10(11):2605-2619. doi: 10.1038/ismej.2016.61. Epub 2016 May 17. ISME J. 2016. PMID: 27187795 Free PMC article.
-
Global Biodiversity of Aquatic Ammonia-Oxidizing Archaea is Partitioned by Habitat.Front Microbiol. 2012 Jul 18;3:252. doi: 10.3389/fmicb.2012.00252. eCollection 2012. Front Microbiol. 2012. PMID: 22826704 Free PMC article.
Cited by
-
Suboxic DOM is bioavailable to surface prokaryotes in a simulated overturn of an oxygen minimum zone, Devil's Hole, Bermuda.Front Microbiol. 2023 Dec 20;14:1287477. doi: 10.3389/fmicb.2023.1287477. eCollection 2023. Front Microbiol. 2023. PMID: 38179459 Free PMC article.
-
Microbial community composition across a coastal hydrological system affected by submarine groundwater discharge (SGD).PLoS One. 2020 Jun 29;15(6):e0235235. doi: 10.1371/journal.pone.0235235. eCollection 2020. PLoS One. 2020. PMID: 32598345 Free PMC article.
-
Deep ocean microbial communities produce more stable dissolved organic matter through the succession of rare prokaryotes.Sci Adv. 2022 Jul 8;8(27):eabn0035. doi: 10.1126/sciadv.abn0035. Epub 2022 Jul 8. Sci Adv. 2022. PMID: 35857452 Free PMC article.
-
Archaeal community variation in the Qinhuangdao coastal aquaculture zone revealed by high-throughput sequencing.PLoS One. 2019 Jun 21;14(6):e0218611. doi: 10.1371/journal.pone.0218611. eCollection 2019. PLoS One. 2019. PMID: 31226149 Free PMC article.
-
Oxidation of sulfur, hydrogen, and iron by metabolically versatile Hydrogenovibrio from deep sea hydrothermal vents.ISME J. 2024 Jan 8;18(1):wrae173. doi: 10.1093/ismejo/wrae173. ISME J. 2024. PMID: 39276367 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

