An update on enzalutamide in the treatment of prostate cancer
- PMID: 25642291
- PMCID: PMC4294802
- DOI: 10.1177/1756287214555336
An update on enzalutamide in the treatment of prostate cancer
Abstract
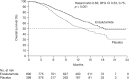
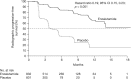
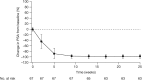
Enzalutamide is an oral androgen receptor inhibitor that targets multiple steps in the androgen receptor signaling pathway. In the randomized phase III AFFIRM study, significant improvements in survival versus placebo were observed when enzalutamide was used as a treatment for patients with metastatic castration-resistant prostate cancer (mCRPC) following prior treatment with docetaxel. Additional benefits included significant delay in time to first skeletal-related event, and improvement in several measures of pain and health-related quality of life. Treatment effects were consistent across all prespecified subgroups. The phase III PREVAIL study evaluated enzalutamide versus placebo in patients with mCRPC who had not received chemotherapy. Enzalutamide significantly decreased the risk of radiographic progression and death. There were also significant improvements in all secondary and prespecified exploratory endpoints, including delayed initiation of chemotherapy, reduction in risk of first skeletal-related event and a high percentage of patients with objective response compared with placebo. Enzalutamide was also studied in hormone naïve patients (as monotherapy) in a small, open-label phase II study in patients with prostate cancer who were eligible for androgen-deprivation therapy. A prostate-specific antigen (PSA) response, defined as ⩾80% decline in PSA level from baseline at week 25, was achieved in 92.5% of patients. Long-term follow up is ongoing. Despite differences between these three trials, enzalutamide displayed a favorable safety profile in all three patient populations. Similar rates of adverse events between the enzalutamide and placebo groups were observed in AFFIRM and PREVAIL, with fatigue, diarrhea, back pain and hot flashes being more common with enzalutamide than with placebo. Hypertension was reported at a higher rate in the enzalutamide group than in the placebo group in PREVAIL. Breast-related disorders associated with enzalutamide treatment were also reported in the Monotherapy trial. Few seizures were reported in any trial. Enzalutamide is being studied in several early disease state populations.
Keywords: AFFIRM trial; PREVAIL trial; efficacy; enzalutamide; prostate cancer; safety.
Conflict of interest statement
Figures
References
-
- Argoud T., Boutroy S., Claustrat B., Chapurlat R., Szulc P. (2014) Association between sex steroid levels and bone microarchitecture in men: the STRAMBO study. J Clin Endocrinol Metab 99: 1400–1410. - PubMed
-
- Astellas Pharma US Inc. and Medivation Inc. (2014a) XTANDI US prescribing information. Available at: http://www.astellas.us/docs/12A005-ENZ-WPI.pdf
-
- Astellas Pharma US Inc. and Medivation Inc. (2014b) XTANDI summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info....
-
- Badrising S., van der Noort V., van Oort I., van den Berg H., Los M., Hamberg P., et al. (2014) Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer 120: 968–975. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

