Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally aging mice
- PMID: 25642732
- PMCID: PMC4476071
- DOI: 10.1021/cn500308h
Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally aging mice
Abstract
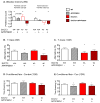
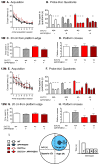
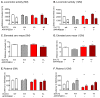
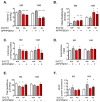
Subclinical vitamin C deficiency is widespread in many populations, but its role in both Alzheimer's disease and normal aging is understudied. In the present study, we decreased brain vitamin C in the APPSWE/PSEN1deltaE9 mouse model of Alzheimer's disease by crossing APP/PSEN1(+) bigenic mice with SVCT2(+/-) heterozygous knockout mice, which have lower numbers of the sodium-dependent vitamin C transporter required for neuronal vitamin C transport. SVCT2(+/-) mice performed less well on the rotarod task at both 5 and 12 months of age compared to littermates. SVCT2(+/-) and APP/PSEN1(+) mice and the combination genotype SVCT2(+/-)APP/PSEN1(+) were also impaired on multiple tests of cognitive ability (olfactory memory task, Y-maze alternation, conditioned fear, Morris water maze). In younger mice, both low vitamin C (SVCT2(+/-)) and APP/PSEN1 mutations increased brain cortex oxidative stress (malondialdehyde, protein carbonyls, F2-isoprostanes) and decreased total glutathione compared to wild-type controls. SVCT2(+/-) mice also had increased amounts of both soluble and insoluble Aβ1-42 and a higher Aβ1-42/1-40 ratio. By 14 months of age, oxidative stress levels were similar among groups, but there were more amyloid-β plaque deposits in both hippocampus and cortex of SVCT2(+/-)APP/PSEN1(+) mice compared to APP/PSEN1(+) mice with normal brain vitamin C. These data suggest that even moderate intracellular vitamin C deficiency plays an important role in accelerating amyloid pathogenesis, particularly during early stages of disease development, and that these effects are likely modulated by oxidative stress pathways.
Keywords: Alzheimer’s disease; Vitamin C; amyloid; cognition; mouse models; oxidative stress.
Conflict of interest statement
Figures

References
-
- Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Archives of neurology. 2002;59(6):972–6. - PubMed
-
- Pratico D, Sung S. Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer's disease. J Alzheimers Dis. 2004;6(2):171–5. - PubMed
-
- Morris MC, Beckett LA, Scherr PA, Hebert LE, Bennett DA, Field TS, Evans DA. Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease. Alzheimer disease and associated disorders. 1998;12(3):121–6. - PubMed
-
- Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Wilson RS, Scherr PA. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. Jama. 2002;287(24):3230–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

