MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis
- PMID: 25642768
- PMCID: PMC4362225
- DOI: 10.1172/JCI74347
MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis
Abstract
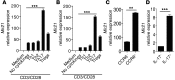
Accumulation of IL-17-producing Th17 cells is associated with the development of multiple autoimmune diseases; however, the contribution of microRNA (miRNA) pathways to the intrinsic control of Th17 development remains unclear. Here, we demonstrated that miR-21 expression is elevated in Th17 cells and that mice lacking miR-21 have a defect in Th17 differentiation and are resistant to experimental autoimmune encephalomyelitis (EAE). Furthermore, we determined that miR-21 promotes Th17 differentiation by targeting and depleting SMAD-7, a negative regulator of TGF-β signaling. Moreover, the decreases in Th17 differentiation in miR-21-deficient T cells were associated with defects in SMAD-2/3 activation and IL-2 suppression. Finally, we found that treatment of WT mice with an anti-miR-21 oligonucleotide reduced the clinical severity of EAE, which was associated with a decrease in Th17 cells. Thus, we have characterized a T cell-intrinsic miRNA pathway that enhances TGF-β signaling, limits the autocrine inhibitory effects of IL-2, and thereby promotes Th17 differentiation and autoimmunity.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

