FOXP3+ regulatory T cell development and function require histone/protein deacetylase 3
- PMID: 25642770
- PMCID: PMC4362235
- DOI: 10.1172/JCI77088
FOXP3+ regulatory T cell development and function require histone/protein deacetylase 3
Erratum in
-
FOXP3⁺ regulatory T cell development and function require histone/protein deacetylase 3.J Clin Invest. 2015 Aug 3;125(8):3304. doi: 10.1172/JCI83084. Epub 2015 Aug 3. J Clin Invest. 2015. PMID: 26237045 Free PMC article. No abstract available.
Abstract
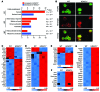
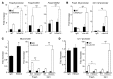
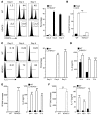
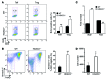
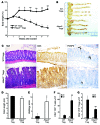
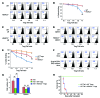
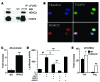
Treg dysfunction is associated with a variety of inflammatory diseases. Treg populations are defined by expression of the oligomeric transcription factor FOXP3 and inability to produce IL-2, a cytokine required for T cell maintenance and survival. FOXP3 activity is regulated post-translationally by histone/protein acetyltransferases and histone/protein deacetylases (HDACs). Here, we determined that HDAC3 mediates both the development and function of the two main Treg subsets, thymus-derived Tregs and induced Tregs (iTregs). We determined that HDAC3 and FOXP3 physically interact and that HDAC3 expression markedly reduces Il2 promoter activity. In murine models, conditional deletion of Hdac3 during thymic Treg development restored Treg production of IL-2 and blocked the suppressive function of Tregs. HDAC3-deficient mice died from autoimmunity by 4-6 weeks of age; however, injection of WT FOXP3+ Tregs prolonged survival. Adoptive transfer of Hdac3-deficient Tregs, unlike WT Tregs, did not control T cell proliferation in naive mice and did not prevent allograft rejection or colitis. HDAC3 also regulated the development of iTregs, as HDAC3-deficient conventional T cells were not converted into iTregs under polarizing conditions and produced large amounts of IL-2, IL-6, and IL-17. We conclude that HDAC3 is essential for the normal development and suppressive functions of thymic and peripheral FOXP3+ Tregs.
Figures


Comment in
-
Histone/protein deacetylase 3 dictates critical aspects of regulatory T cell development and function.Cell Mol Immunol. 2016 Jul;13(4):415-7. doi: 10.1038/cmi.2015.51. Epub 2015 Jun 15. Cell Mol Immunol. 2016. PMID: 26073467 Free PMC article. No abstract available.
Similar articles
-
Histone/protein deacetylase 11 targeting promotes Foxp3+ Treg function.Sci Rep. 2017 Aug 17;7(1):8626. doi: 10.1038/s41598-017-09211-3. Sci Rep. 2017. PMID: 28819166 Free PMC article.
-
Foxp3+ T-regulatory cells require DNA methyltransferase 1 expression to prevent development of lethal autoimmunity.Blood. 2013 May 2;121(18):3631-9. doi: 10.1182/blood-2012-08-451765. Epub 2013 Feb 26. Blood. 2013. PMID: 23444399 Free PMC article.
-
T-regulatory cells require Sin3a for stable expression of Foxp3.Front Immunol. 2024 Aug 2;15:1444937. doi: 10.3389/fimmu.2024.1444937. eCollection 2024. Front Immunol. 2024. PMID: 39156895 Free PMC article.
-
Histone Deacetylation Inhibitors as Modulators of Regulatory T Cells.Int J Mol Sci. 2020 Mar 29;21(7):2356. doi: 10.3390/ijms21072356. Int J Mol Sci. 2020. PMID: 32235291 Free PMC article. Review.
-
Induced Regulatory T Cells: Their Development, Stability, and Applications.Trends Immunol. 2016 Nov;37(11):803-811. doi: 10.1016/j.it.2016.08.012. Epub 2016 Sep 9. Trends Immunol. 2016. PMID: 27623114 Review.
Cited by
-
Dual inhibition of AKT-mTOR and AR signaling by targeting HDAC3 in PTEN- or SPOP-mutated prostate cancer.EMBO Mol Med. 2018 Apr;10(4):e8478. doi: 10.15252/emmm.201708478. EMBO Mol Med. 2018. PMID: 29523594 Free PMC article.
-
Reprogramming of regulatory T cells in inflammatory tumor microenvironment: can it become immunotherapy turning point?Front Immunol. 2024 Feb 21;15:1345838. doi: 10.3389/fimmu.2024.1345838. eCollection 2024. Front Immunol. 2024. PMID: 38449875 Free PMC article. Review.
-
Histone Deacetylases and Cardiometabolic Diseases.Arterioscler Thromb Vasc Biol. 2015 Sep;35(9):1914-9. doi: 10.1161/ATVBAHA.115.305046. Epub 2015 Jul 16. Arterioscler Thromb Vasc Biol. 2015. PMID: 26183616 Free PMC article. Review.
-
Efferocytosis in dendritic cells: an overlooked immunoregulatory process.Front Immunol. 2024 May 21;15:1415573. doi: 10.3389/fimmu.2024.1415573. eCollection 2024. Front Immunol. 2024. PMID: 38835772 Free PMC article. Review.
-
Molecular and physiological manifestations and measurement of aging in humans.Aging Cell. 2017 Aug;16(4):624-633. doi: 10.1111/acel.12601. Epub 2017 May 23. Aging Cell. 2017. PMID: 28544158 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

