Human-derived neural progenitors functionally replace astrocytes in adult mice
- PMID: 25642771
- PMCID: PMC4362241
- DOI: 10.1172/JCI69097
Human-derived neural progenitors functionally replace astrocytes in adult mice
Abstract
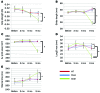
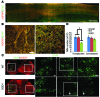
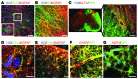
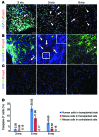
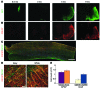
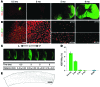
Astrocytes are integral components of the homeostatic neural network as well as active participants in pathogenesis of and recovery from nearly all neurological conditions. Evolutionarily, compared with lower vertebrates and nonhuman primates, humans have an increased astrocyte-to-neuron ratio; however, a lack of effective models has hindered the study of the complex roles of human astrocytes in intact adult animals. Here, we demonstrated that after transplantation into the cervical spinal cords of adult mice with severe combined immunodeficiency (SCID), human pluripotent stem cell-derived (PSC-derived) neural progenitors migrate a long distance and differentiate to astrocytes that nearly replace their mouse counterparts over a 9-month period. The human PSC-derived astrocytes formed networks through their processes, encircled endogenous neurons, and extended end feet that wrapped around blood vessels without altering locomotion behaviors, suggesting structural, and potentially functional, integration into the adult mouse spinal cord. Furthermore, in SCID mice transplanted with neural progenitors derived from induced PSCs from patients with ALS, astrocytes were generated and distributed to a similar degree as that seen in mice transplanted with healthy progenitors; however, these mice exhibited motor deficit, highlighting functional integration of the human-derived astrocytes. Together, these results indicate that this chimeric animal model has potential for further investigating the roles of human astrocytes in disease pathogenesis and repair.
Figures
Similar articles
-
Sporadic ALS Astrocytes Induce Neuronal Degeneration In Vivo.Stem Cell Reports. 2017 Apr 11;8(4):843-855. doi: 10.1016/j.stemcr.2017.03.003. Epub 2017 Mar 30. Stem Cell Reports. 2017. PMID: 28366455 Free PMC article.
-
Human neural progenitors differentiate into astrocytes and protect motor neurons in aging rats.Exp Neurol. 2016 Jun;280:41-9. doi: 10.1016/j.expneurol.2016.03.023. Epub 2016 Mar 29. Exp Neurol. 2016. PMID: 27032721
-
Focal transplantation of human iPSC-derived glial-rich neural progenitors improves lifespan of ALS mice.Stem Cell Reports. 2014 Aug 12;3(2):242-9. doi: 10.1016/j.stemcr.2014.05.017. Epub 2014 Jun 26. Stem Cell Reports. 2014. PMID: 25254338 Free PMC article.
-
Crosstalk between astrocytes and motor neurons: what is the message?Exp Neurol. 2008 May;211(1):1-6. doi: 10.1016/j.expneurol.2008.01.008. Epub 2008 Jan 26. Exp Neurol. 2008. PMID: 18291372 Review.
-
[Neuronal cells derived from induced pluripotent stem cells to model motoneuron diseases].Biol Aujourdhui. 2016;210(1):27-36. doi: 10.1051/jbio/2016004. Epub 2016 Jun 10. Biol Aujourdhui. 2016. PMID: 27286578 Review. French.
Cited by
-
NR1D1 downregulation in astrocytes induces a phenotype that is detrimental to cocultured motor neurons.FASEB J. 2022 Apr;36(4):e22262. doi: 10.1096/fj.202101275R. FASEB J. 2022. PMID: 35319791 Free PMC article.
-
Amelioration of Amyotrophic Lateral Sclerosis in SOD1G93A Mice by M2 Microglia from Transplanted Marrow.In Vivo. 2019 May-Jun;33(3):675-688. doi: 10.21873/invivo.11526. In Vivo. 2019. PMID: 31028184 Free PMC article.
-
A Defined and Scalable Peptide-Based Platform for the Generation of Human Pluripotent Stem Cell-Derived Astrocytes.ACS Biomater Sci Eng. 2020 Jun 8;6(6):3477-3490. doi: 10.1021/acsbiomaterials.0c00067. Epub 2020 May 6. ACS Biomater Sci Eng. 2020. PMID: 32550261 Free PMC article.
-
Recovery of Neurovascular Unit Integrity by CDK5-KD Astrocyte Transplantation in a Global Cerebral Ischemia Model.Mol Neurobiol. 2018 Nov;55(11):8563-8585. doi: 10.1007/s12035-018-0992-1. Epub 2018 Mar 22. Mol Neurobiol. 2018. PMID: 29564811
-
Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration.Nat Med. 2016 May;22(5):479-87. doi: 10.1038/nm.4066. Epub 2016 Mar 28. Nat Med. 2016. PMID: 27019328 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

