The role of phosphate in a multistep enzymatic reaction: reactions of the substrate and intermediate in pieces
- PMID: 25642788
- PMCID: PMC4507815
- DOI: 10.1021/ja512911f
The role of phosphate in a multistep enzymatic reaction: reactions of the substrate and intermediate in pieces
Abstract
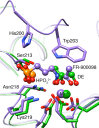
Several mechanistically unrelated enzymes utilize the binding energy of their substrate's nonreacting phosphoryl group to accelerate catalysis. Evidence for the involvement of the phosphodianion in transition state formation has come from reactions of the substrate in pieces, in which reaction of a truncated substrate lacking its phosphorylmethyl group is activated by inorganic phosphite. What has remained unknown until now is how the phosphodianion group influences the reaction energetics at different points along the reaction coordinate. 1-Deoxy-D-xylulose-5-phosphate (DXP) reductoisomerase (DXR), which catalyzes the isomerization of DXP to 2-C-methyl-D-erythrose 4-phosphate (MEsP) and subsequent NADPH-dependent reduction, presents a unique opportunity to address this concern. Previously, we have reported the effect of covalently linked phosphate on the energetics of DXP turnover. Through the use of chemically synthesized MEsP and its phosphate-truncated analogue, 2-C-methyl-D-glyceraldehyde, the current study revealed a loss of 6.1 kcal/mol of kinetic barrier stabilization upon truncation, of which 4.4 kcal/mol was regained in the presence of phosphite dianion. The activating effect of phosphite was accompanied by apparent tightening of its interactions within the active site at the intermediate stage of the reaction, suggesting a role of the phosphodianion in disfavoring intermediate release and in modulation of the on-enzyme isomerization equilibrium. The results of kinetic isotope effect and structural studies indicate rate limitation by physical steps when the covalent linkage is severed. These striking differences in the energetics of the natural reaction and the reactions in pieces provide a deeper insight into the contribution of enzyme-phosphodianion interactions to the reaction coordinate.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


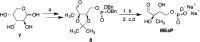



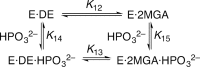
Similar articles
-
DXP reductoisomerase: reaction of the substrate in pieces reveals a catalytic role for the nonreacting phosphodianion group.Biochemistry. 2013 Apr 2;52(13):2302-8. doi: 10.1021/bi400092n. Epub 2013 Mar 20. Biochemistry. 2013. PMID: 23473304
-
Binding energy and catalysis by D-xylose isomerase: kinetic, product, and X-ray crystallographic analysis of enzyme-catalyzed isomerization of (R)-glyceraldehyde.Biochemistry. 2011 Nov 22;50(46):10170-81. doi: 10.1021/bi201378c. Epub 2011 Oct 27. Biochemistry. 2011. PMID: 21995300 Free PMC article.
-
Isoprenoid biosynthesis via the methylerythritol phosphate pathway. Mechanistic investigations of the 1-deoxy-D-xylulose 5-phosphate reductoisomerase.Eur J Biochem. 2002 Sep;269(18):4446-57. doi: 10.1046/j.1432-1033.2002.03150.x. Eur J Biochem. 2002. PMID: 12230556
-
Specificity in transition state binding: the Pauling model revisited.Biochemistry. 2013 Mar 26;52(12):2021-35. doi: 10.1021/bi301491r. Epub 2013 Feb 4. Biochemistry. 2013. PMID: 23327224 Free PMC article. Review.
-
Mechanism and inhibition of 1-deoxy-D-xylulose-5-phosphate reductoisomerase.Bioorg Chem. 2014 Dec;57:171-185. doi: 10.1016/j.bioorg.2014.06.001. Epub 2014 Jun 19. Bioorg Chem. 2014. PMID: 24998420 Review.
Cited by
-
Phosphorus Kβ X-ray emission spectroscopy detects non-covalent interactions of phosphate biomolecules in situ.Chem Sci. 2021 Apr 29;12(22):7888-7901. doi: 10.1039/d1sc01266e. Chem Sci. 2021. PMID: 34168842 Free PMC article.
-
Utilization of Cofactor Binding Energy for Enzyme Catalysis: Formate Dehydrogenase-Catalyzed Reactions of the Whole NAD Cofactor and Cofactor Pieces.Biochemistry. 2023 Aug 1;62(15):2314-2324. doi: 10.1021/acs.biochem.3c00290. Epub 2023 Jul 18. Biochemistry. 2023. PMID: 37463347 Free PMC article.
-
Glycerol 3-Phosphate Dehydrogenase: Role of the Protein Conformational Change in Activation of a Readily Reversible Enzyme-Catalyzed Hydride Transfer Reaction.Biochemistry. 2024 Apr 16;63(8):1016-1025. doi: 10.1021/acs.biochem.3c00702. Epub 2024 Mar 28. Biochemistry. 2024. PMID: 38546289 Free PMC article.
-
A reevaluation of the origin of the rate acceleration for enzyme-catalyzed hydride transfer.Org Biomol Chem. 2017 Oct 31;15(42):8856-8866. doi: 10.1039/c7ob01652b. Org Biomol Chem. 2017. PMID: 28956050 Free PMC article. Review.
-
Modeling the Role of a Flexible Loop and Active Site Side Chains in Hydride Transfer Catalyzed by Glycerol-3-phosphate Dehydrogenase.ACS Catal. 2020 Oct 2;10(19):11253-11267. doi: 10.1021/acscatal.0c02757. Epub 2020 Sep 3. ACS Catal. 2020. PMID: 33042609 Free PMC article.
References
-
- Koumbis A. E.; Kaitaidis A. D.; Kotoulas S. S. Tetrahedron Lett. 2006, 47, 8479.
-
- Kis K.; Wungsintaweekul J.; Eisenreich W.; Zenk M. H.; Bacher A. J. Org. Chem. 2000, 65, 587. - PubMed
-
- Smith N. D.; Goodman M. Org. Lett. 2003, 5, 1035. - PubMed
-
- Avenoza A.; Cativiela C.; Corzana F.; Peregrina J. M.; Sucunza D.; Zurbano M. M. Tetrahedron: Asymmetry 2001, 12, 949.
-
- Bennani Y. L.; Sharpless K. B. Tetrahedron Lett. 1993, 34, 2079.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

