Influence of lipopolysaccharide-binding protein on pulmonary inflammation in gram-negative pneumonia
- PMID: 25643011
- PMCID: PMC4433570
- DOI: 10.1097/SHK.0000000000000349
Influence of lipopolysaccharide-binding protein on pulmonary inflammation in gram-negative pneumonia
Abstract
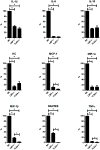
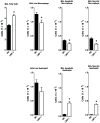
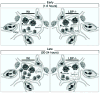
Lipopolysaccharide-binding protein (LBP) is upregulated as part of the acute-phase response. Lipopolysaccharide-binding protein has a known multifunctional role in potentiating the recognition, clearance, and killing of gram-negative bacteria. In a Klebsiella pneumonia model, we previously demonstrated that LBP gene-deficient mice (LBP-/-) mice experience increased mortality when compared with wild-type (Wt) mice (98% vs. 59%). We hypothesize that LBP is essential to bacterial clearance from the lung, and its absence leads to alteration of the pulmonary inflammatory response to pneumonia. Twelve- to 16-week-old female C57Bl/6 Wt mice and age-matched LBP-/- mice were administered 1 × 10(3) colony-forming units of Klebsiella pneumoniae by intratracheal injection. Animals were euthanized at 6, 12, 24, or 36 h after inoculation. Lung tissue and bronchoalveolar lavage samples were obtained. Lung homogenate samples were assayed to determine quantitative bacterial load per whole lung, proinflammatory cytokine concentrations, myeloperoxidase activity, and assessment of pulmonary leukocyte populations. In vitro production of inflammatory mediators were also assayed after LPS stimulation of peritoneal macrophages isolated from Wt, Toll-like receptor 4 (TLR4)-deficient, and LBP-/- mice. The LBP-/- mice demonstrated significantly elevated levels of bacteria in the lung at 24 and 36 h when compared with Wt controls. The average lung levels of proinflammatory cytokines interleukin-1β (IL-1β), IL-6, keratinocyte-derived chemokine, and macrophage-inflammatory protein-2 were greater in the LBP mice and remained elevated longer when compared with those in the Wt mice. Myeloperoxidase activity, an indicator of neutrophil content, was significantly increased at time 36 h in the LBP mice. After in vitro stimulation of peritoneal macrophages with LPS, production of IL-1β, IL-6, IL-10, keratinocyte-derived chemokine, and macrophage-inflammatory protein-1α were suppressed in LBP and TLR4-deficient mice compared with that in Wt. Absence of a functional LBP-/- gene results in diminished clearance of gram-negative bacteria from the pulmonary system. Failure to recognize and clear gram-negative bacteria via the LBP/TLR4 axis results in an initial delayed inflammatory response. This delay in LBP-/- mice is followed by excessive amplification and prolonged elevation of proinflammatory mediators and neutrophil sequestration within the lungs.
Conflict of interest statement
Conflict of Interest Disclosure: None.
Figures
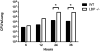
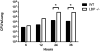


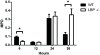
References
-
- Branger J, Florquin S, Knapp S, Leemans JC, Pater JM, Speelman P, Golenbock DT, van der Poll T. LPS-binding protein-deficient mice have an impaired defense against Gram-negative but not Gram-positive pneumonia. Int Immunol. 2004;16:1605–1611. - PubMed
-
- Dentener MA, Vreugdenhil AC, Hoet PH, Vernooy JH, Nieman FH, Heumann D, Janssen YM, Buurman WA, Wouters EF. Production of the acute-phase protein lipopolysaccharide-binding protein by respiratory type II epithelial cells: implications for local defense to bacterial endotoxins. Am J Respir Cell Mol Biol. 2000;23:146–153. - PubMed
-
- Fan MH, Klein RD, Steinstraesser L, Merry AC, Nemzek JA, Remick DG, Wang SC, Su GL. An essential role for lipopolysaccharide-binding protein in pulmonary innate immune responses. Shock. 2002;18:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

