Efficacy and safety of transcutaneous electrical acupoint stimulation to treat muscle spasticity following brain injury: a double-blinded, multicenter, randomized controlled trial
- PMID: 25643051
- PMCID: PMC4314074
- DOI: 10.1371/journal.pone.0116976
Efficacy and safety of transcutaneous electrical acupoint stimulation to treat muscle spasticity following brain injury: a double-blinded, multicenter, randomized controlled trial
Abstract
Objective: This study was aimed at evaluating the clinical efficacy and safety of transcutaneous electrical acupoint stimulation (TEAS) to treat muscle spasticity after brain injury (Chinese Clinical Trial Registry: ChiCTR-TRC-11001310).
Methods: A total of 60 patients with muscle spasticity after brain injury were randomized to the following 3 groups: 100, 2, and 0 Hz (sham) TEAS. The acupoints Hegu (LI4)--Yuji (LU10) and Zusanli (ST36)--Chengshan (BL57) on the injured side were stimulated at 0, 2, or 100 Hz, 5 times per week for 4 weeks. The patients were followed up for 1 and 2 months after the treatments. The effects of the treatments on muscle spasticity at the wrist, thumb, the other 4 fingers, elbow, shoulder, knee, and ankle were evaluated by the Modified Ashworth Scale, and the effects on disability were assessed by the Disability Assessment Scale. The walking capability was evaluated by the Holden functional ambulation classification score. The overall performance was assessed by the Global Assessment Scale score and the improved Barthel Index. The safety of the treatments administered was also monitored.
Results: The wrist spasticity was significantly reduced from baseline at weeks 2, 3, and 4 of treatment and at the 1- and 2-month follow-up visits in the 100 Hz group (P < 0.01). Compared with 2 Hz or sham TEAS, 100 Hz TEAS decreased wrist spasticity at weeks 2, 3, and 4 of treatment and 1 month after treatment (P < 0.001). The other endpoints were not affected by the treatments. No treatment-emergent adverse events were reported during treatments and follow-up visits.
Conclusions: TEAS appears to be a safe and effective therapy to relieve muscle spasticity after brain injury, although large-scale studies are required to further verify the findings.
Trial registration: Chinese Clinical Trial Registry ChiCTR-TRC-11001310 http://www.chictr.org.
Conflict of interest statement
Figures
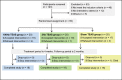


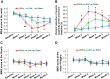
References
-
- Lance JW (1980) Symposium synopsis. In: Feldman RG, Young RR, Koella WP, editors. Spasticity: Disordered Motor Control. Chicago: Year Book Medical Publishers; 485 p.
-
- Van Kuijk AA, Hendricks HT, Pasman JW, Kremer BH, Geurts AC (2007) Are clinical characteristics associated with upper-extremity hypertonia in severe ischaemic supratentorial stroke? J Rehab Med 39: 33–37. - PubMed
-
- Ward AB (2012) A literature review of the pathophysiology and onset of post-stroke spasticity. Euro J Neurol 19: 21–27. - PubMed
-
- Bhakta BB (2000) Management of spasticity in stroke. Brit Med Bull 56: 476–485. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

