Upper limb strength and function changes during a one-year follow-up in non-ambulant patients with Duchenne Muscular Dystrophy: an observational multicenter trial
- PMID: 25643053
- PMCID: PMC4314080
- DOI: 10.1371/journal.pone.0113999
Upper limb strength and function changes during a one-year follow-up in non-ambulant patients with Duchenne Muscular Dystrophy: an observational multicenter trial
Abstract
Introduction: Upper limb evaluation of patients with Duchenne Muscular Dystrophy is crucially important to evaluations of efficacy of new treatments in non-ambulant patients. In patients who have lost ambulation, there are few validated and informative outcome measures. In addition, longitudinal data demonstrating sensitivity to clinical evolution of outcome measures over short-term periods are lacking.
Patients and methods: We report here the results of a one-year multicenter study using specifically designed tools to assess grip, pinch strength, and hand function in wheelchair-bound patients. Our study assessed 53 non-ambulant patients with Duchenne muscular dystrophy aged 17.1 ± 4.8 years (range: 9 - 28.1 years). The average Brooke functional score of these patients was 4.6 ± 1.1. The average forced vital capacity was 44.5% predicted and 19 patients used non-invasive ventilation. Patients were assessed at baseline, 6 months, and one year using the Motor Function Measure and innovative devices (namely the MyoSet composed of MyoGrip, MyoPinch, and MoviPlate).
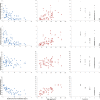
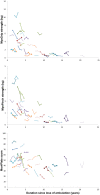
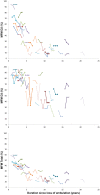
Results: Our study confirmed preliminary data previously reported regarding feasibility of use and of reliability of the MyoSet and the correlation at baseline between distal strength and clinical outcomes such as FVC, Brooke score, age, and duration since loss of ambulation. A significant correlation was observed between the distal upper limb strength and clinical variables. The sensitive dynamometers (MyoGrip and MyoPinch) and MoviPlate captured a 12-month change in non-ambulant Duchenne muscular dystrophy patients of all ages.
Trial registration: ClinicalTrials.gov NCT00993161 NCT00993161.
Conflict of interest statement
Figures
References
-
- Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, et al. (2011) Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet 378: 595–605. 10.1016/S0140-6736(11)60756-3 - DOI - PMC - PubMed
-
- Mendell J, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, et al. (2013) Eteplirsen for the treatment of duchenne muscular dystrophy. Ann Neurol. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

