Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation
- PMID: 25643172
- PMCID: PMC5019199
- DOI: 10.1038/nchembio.1750
Lipid vesicles trigger α-synuclein aggregation by stimulating primary nucleation
Abstract
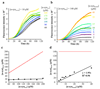
α-Synuclein (α-syn) is a 140-residue intrinsically disordered protein that is involved in neuronal and synaptic vesicle plasticity, but its aggregation to form amyloid fibrils is the hallmark of Parkinson's disease (PD). The interaction between α-syn and lipid surfaces is believed to be a key feature for mediation of its normal function, but under other circumstances it is able to modulate amyloid fibril formation. Using a combination of experimental and theoretical approaches, we identify the mechanism through which facile aggregation of α-syn is induced under conditions where it binds a lipid bilayer, and we show that the rate of primary nucleation can be enhanced by three orders of magnitude or more under such conditions. These results reveal the key role that membrane interactions can have in triggering conversion of α-syn from its soluble state to the aggregated state that is associated with neurodegeneration and to its associated disease states.
Conflict of interest statement
The authors declare they have no competing interests as defined by Nature Publishing Group, or other interests that might be perceived to influence the results and discussion reported in this paper.
Figures





Comment in
-
Protein aggregation: close encounters of the greasy kind.Nat Chem Biol. 2015 Mar;11(3):176-7. doi: 10.1038/nchembio.1759. Nat Chem Biol. 2015. PMID: 25689332 No abstract available.
References
-
- Bellucci A, Navarria L, Zaltieri M, Missale C, Spano P. Alpha-synuclein synaptic pathology and its implications in the development of novel therapeutic approaches to cure Parkinson's disease. Brain Res. 2012;1432:95–113. - PubMed
-
- Bellucci A, et al. From alpha-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson's disease. Brain Res. 2012;1476:183–202. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–66. - PubMed
-
- Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–32. - PubMed
-
- Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol. 2014;15:384–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

