Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo
- PMID: 25643235
- PMCID: PMC4342728
- DOI: 10.1021/nn504905q
Polymeric nanoparticles for nonviral gene therapy extend brain tumor survival in vivo
Abstract
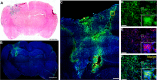
Biodegradable polymeric nanoparticles have the potential to be safer alternatives to viruses for gene delivery; however, their use has been limited by poor efficacy in vivo. In this work, we synthesize and characterize polymeric gene delivery nanoparticles and evaluate their efficacy for DNA delivery of herpes simplex virus type I thymidine kinase (HSVtk) combined with the prodrug ganciclovir (GCV) in a malignant glioma model. We investigated polymer structure for gene delivery in two rat glioma cell lines, 9L and F98, to discover nanoparticle formulations more effective than the leading commercial reagent Lipofectamine 2000. The lead polymer structure, poly(1,4-butanediol diacrylate-co-4-amino-1-butanol) end-modified with 1-(3-aminopropyl)-4-methylpiperazine, is a poly(β-amino ester) (PBAE) and formed nanoparticles with HSVtk DNA that were 138 ± 4 nm in size and 13 ± 1 mV in zeta potential. These nanoparticles containing HSVtk DNA showed 100% cancer cell killing in vitro in the two glioma cell lines when combined with GCV exposure, while control nanoparticles encoding GFP maintained robust cell viability. For in vivo evaluation, tumor-bearing rats were treated with PBAE/HSVtk infusion via convection-enhanced delivery (CED) in combination with systemic administration of GCV. These treated animals showed a significant benefit in survival (p = 0.0012 vs control). Moreover, following a single CED infusion, labeled PBAE nanoparticles spread completely throughout the tumor. This study highlights a nanomedicine approach that is highly promising for the treatment of malignant glioma.
Keywords: DNA; brain tumor; gene therapy; nanomedicine; nonviral gene delivery; polymer.
Figures






References
-
- McGirt M. J.; Chaichana K. L.; Gathinji M.; Attenello F. J.; Than K.; Olivi A.; Weingart J. D.; Brem H.; Quinones-Hinojosa A. R. Independent Association of Extent of Resection with Survival in Patients with Malignant Brain Astrocytoma. J. Neurosurg. 2009, 110, 156–162. - PubMed
-
- Stupp R.; Mason W. P.; van den Bent M. J.; Weller M.; Fisher B.; Taphoorn M. J. B.; Belanger K.; Brandes A. A.; Marosi C.; Bogdahn U.; et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

