Antimicrobial protein and Peptide concentrations and activity in human breast milk consumed by preterm infants at risk of late-onset neonatal sepsis
- PMID: 25643281
- PMCID: PMC4314069
- DOI: 10.1371/journal.pone.0117038
Antimicrobial protein and Peptide concentrations and activity in human breast milk consumed by preterm infants at risk of late-onset neonatal sepsis
Abstract
Objective: We investigated the levels and antimicrobial activity of antimicrobial proteins and peptides (AMPs) in breast milk consumed by preterm infants, and whether deficiencies of these factors were associated with late-onset neonatal sepsis (LOS), a bacterial infection that frequently occurs in preterm infants in the neonatal period.
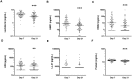
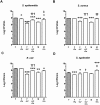
Study design: Breast milk from mothers of preterm infants (≤ 32 weeks gestation) was collected on days 7 (n = 88) and 21 (n = 77) postpartum. Concentrations of lactoferrin, LL-37, beta-defensins 1 and 2, and alpha-defensin 5 were measured by enzyme-linked immunosorbent assay. The antimicrobial activity of breast milk samples against Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, and Streptococcus agalactiae was compared to the activity of infant formula, alone or supplemented with physiological levels of AMPs. Samples of breast milk fed to infants with and without subsequent LOS were compared for levels of AMPs and inhibition of bacterial growth.
Results: Levels of most AMPs and antibacterial activity in preterm breast milk were higher at day 7 than at day 21. Lactoferrin was the only AMP that limited pathogen growth >50% when added to formula at a concentration equivalent to that present in breast milk. Levels of AMPs were similar in the breast milk fed to infants with and without LOS, however, infants who developed LOS consumed significantly less breast milk and lower doses of milk AMPs than those who were free from LOS.
Conclusions: The concentrations of lactoferrin and defensins in preterm breast milk have antimicrobial activity against common neonatal pathogens.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

