CXCL9 contributes to antimicrobial protection of the gut during citrobacter rodentium infection independent of chemokine-receptor signaling
- PMID: 25643352
- PMCID: PMC4333760
- DOI: 10.1371/journal.ppat.1004648
CXCL9 contributes to antimicrobial protection of the gut during citrobacter rodentium infection independent of chemokine-receptor signaling
Abstract
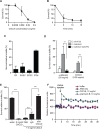
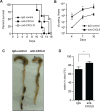
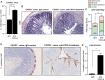
Chemokines have been shown to be effective bactericidal molecules against a variety of bacteria and fungi in vitro. These direct antimicrobial effects are independent of their chemotactic activities involving immunological receptors. However, the direct biological role that these proteins may play in host defense, particularly against intestinal pathogens, is poorly understood. Here, we show that CXCL9, an ELR- chemokine, exhibits direct antimicrobial activity against Citrobacter rodentium, an attaching/effacing pathogen that infects the gut mucosa. Inhibition of this antimicrobial activity in vivo using anti-CXCL9 antibodies increases host susceptibility to C. rodentium infection with pronounced bacterial penetration into crypts, increased bacterial load, and worsened tissue pathology. Using Rag1(-/-) mice and CXCR3(-/-) mice, we demonstrate that the role for CXCL9 in protecting the gut mucosa is independent of an adaptive response or its immunological receptor, CXCR3. Finally, we provide evidence that phagocytes function in tandem with NK cells for robust CXCL9 responses to C. rodentium. These findings identify a novel role for the immune cell-derived CXCL9 chemokine in directing a protective antimicrobial response in the intestinal mucosa.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Yang D (2003) Many chemokines including CCL20/MIP-3 display antimicrobial activity. J Leukocyte Biol 74: 448–455. - PubMed
-
- Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, et al. (2001) Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol 167: 623–627. - PubMed
-
- Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24: 1551–1557. - PubMed
-
- Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415: 389–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

