Silencing dishevelled-1 sensitizes paclitaxel-resistant human ovarian cancer cells via AKT/GSK-3β/β-catenin signalling
- PMID: 25643607
- PMCID: PMC6495546
- DOI: 10.1111/cpr.12161
Silencing dishevelled-1 sensitizes paclitaxel-resistant human ovarian cancer cells via AKT/GSK-3β/β-catenin signalling
Abstract
Objectives: Expression of dishevelled-1 (DVL1) has recently been linked to cancer progression, however, its role in resistance to cancer therapy is unclear. In this study, we aimed to explore the function of DVL1 in paclitaxel-resistant human ovarian cancer cells.
Materials and methods: The MTT assay was used to assess effects of DVL1 silencing on sensitivity of cells that were otherwise resistant to paclitaxel (Taxol). Western blotting and immunofluorescence staining were used to examine effects of DVL1 on AKT/GSK-3β/β-catenin signalling.
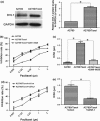
Results: Dishevelled-1 was found to be over-expressed in a paclitaxel-resistant cell line derived from human ovarian cancer cell line A2780 (A2780/Taxol line) as well as parental A2780 cells. Down-regulation of DVL1 (using the inhibitor 3289-8625 or siRNA (siDVL1) against DVL1) sensitized A2780/Taxol cells to paclitaxel. Over-expression of DVL1 in A2780 cells increased protein levels of P-gp, BCRP and Bcl-2, which are known targets of β-catenin. Silencing DVL1 in A2780/Taxol cells also reduced levels of these proteins, and led to accumulation of β-catenin. In addition, DVL1 aberrantly activated AKT/GSK-3β/β-catenin signalling. Inactivation of AKT signalling attenuated DVL1-mediated inhibition of GSK-3β and accumulation of β-catenin, in both A2780 and A2780/Taxol cells.
Conclusions: Taken together, these results suggest that silencing DVL1 sensitized A2780/Taxol cells to paclitaxel, by down-regulating AKT/GSK-3β/β-catenin signalling, providing a novel strategy for chemosensitization of ovarian cancer to paclitaxel-induced cytotoxicity.
© 2015 John Wiley & Sons Ltd.
Figures



References
-
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108. - PubMed
-
- Permuth‐Wey J, Sellers TA (2009) Epidemiology of ovarian cancer. Methods Mol. Biol. 472, 413–437. - PubMed
-
- Agarwal R, Kaye SB (2003) Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 3, 502–516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

