rhErythropoietin-b as a tissue protective agent in kidney transplantation: a pilot randomized controlled trial
- PMID: 25643790
- PMCID: PMC4330593
- DOI: 10.1186/s13104-014-0964-0
rhErythropoietin-b as a tissue protective agent in kidney transplantation: a pilot randomized controlled trial
Abstract
Background: Extended criteria donor (ECD) and donation after circulatory death (DCD) kidneys are at increased risk of delayed graft function (DGF). Experimental evidence suggests that erythropoietin (EPO) attenuates renal damage in acute kidney injury. This study piloted the administration of high dose recombinant human EPO-beta at implantation of ECD and DCD kidneys, and evaluated biomarkers of kidney injury post-transplant.
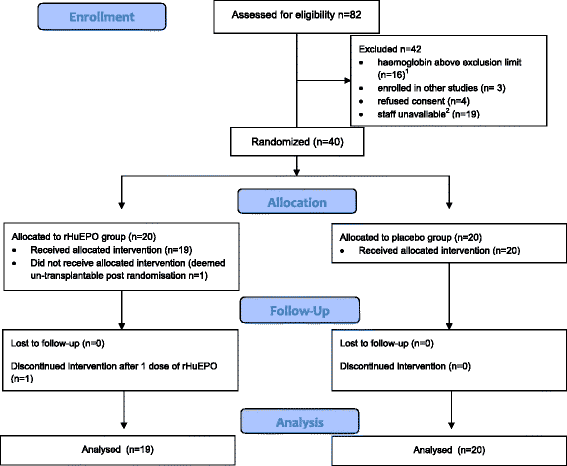
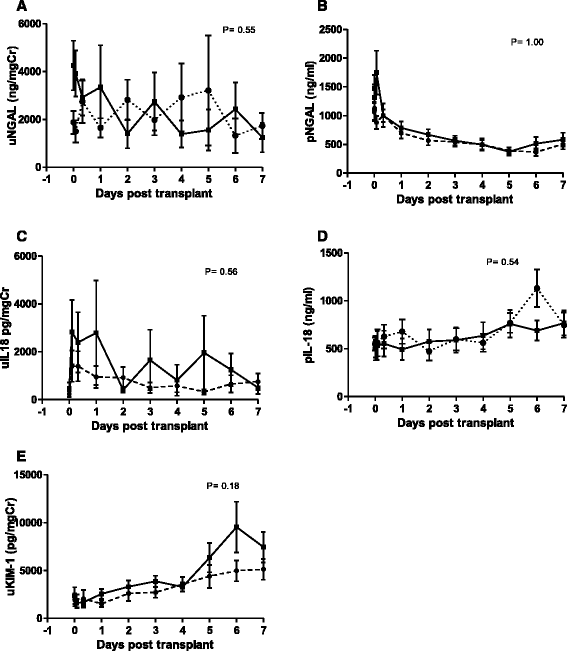
Methods: Forty patients were randomly assigned to receive either rhEPO-b (100,000 iu) (n = 19 in the intervention group, as 1 patient was un-transplantable post randomisation), or placebo (n = 20) in this, double blind, placebo-controlled trial at Manchester Royal Infirmary from August 2007 to June 2009. Participants received either an ECD (n = 17) or DCD (n = 22) kidney. Adverse events, renal function, haematopoietic markers, and rejections were recorded out to 90 days post-transplant. Biomarkers of kidney injury (neutrophil gelatinase-associated lipocalin, Kidney Injury Molecule-1 and IL-18) were measured in blood and urine during the first post-operative week.
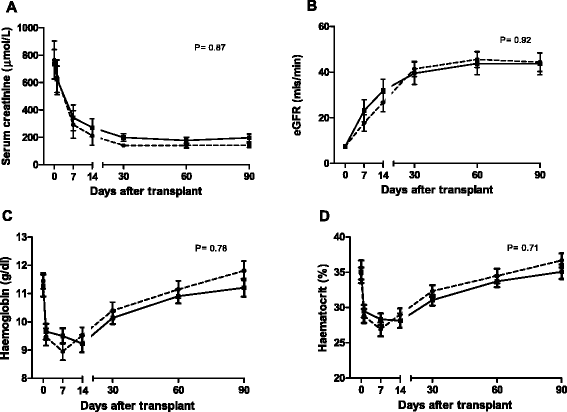
Results: The incidence of DGF (53% vs 55%) (RR = 1.0; CI = 0.5-1.6; p = 0.93) and slow graft function (SGF) (32% vs 25%) (RR = 1.1; CI = 0.5-1.9; p = 0.73) respectively, serum creatinine, eGFR, haemoglobin and haematocrit, blood pressure, and acute rejection were similar in the 2 study arms. High dose rhEPO-b had little effect on the temporal profiles of the biomarkers.
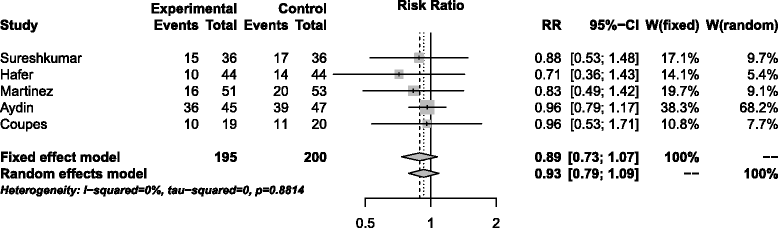
Conclusions: High dose rhEPO-b appears to be safe and well tolerated in the early post- transplant period in this study, but has little effect on delayed or slow graft function in recipients of kidneys from DCD and ECD donors. Comparing the profiles of biomarkers of kidney injury (NGAL, IL-18 and KIM-1) showed little difference between the rhEPO-b treated and placebo groups. A meta-analysis of five trials yielded an overall estimate of the RR for DGF of 0.89 (CI = 0.73; 1.07), a modest effect favouring EPO but not a significant difference. A definitive trial based on this estimate would require 1000-2500 patients per arm for populations with base DGF rates of 50-30% and 90% power. Such a trial is clearly unfeasible.
Trial registration: EudraCT Number 2006-005373-22 ISRCTN ISRCTN85447324 registered 19/08/09.
Figures
Similar articles
-
The early diagnosis of acute renal graft dysfunction: a challenge we face. The role of novel biomarkers.Ann Transplant. 2011 Jan-Mar;16(1):90-8. Ann Transplant. 2011. PMID: 21436782 Review.
-
Is plasma and urine neutrophil gelatinase-associated lipocalin (NGAL) determination in donors and recipients predictive of renal function after kidney transplantation?Clin Biochem. 2014 Oct;47(15):68-72. doi: 10.1016/j.clinbiochem.2014.06.079. Epub 2014 Jul 8. Clin Biochem. 2014. PMID: 25011070
-
Effect of high-dose erythropoietin on graft function after kidney transplantation: a randomized, double-blind clinical trial.Clin J Am Soc Nephrol. 2012 Sep;7(9):1498-506. doi: 10.2215/CJN.01360212. Epub 2012 Jun 28. Clin J Am Soc Nephrol. 2012. PMID: 22745272 Free PMC article. Clinical Trial.
-
Pre-transplant Evaluation of Donor Urinary Biomarkers can Predict Reduced Graft Function After Deceased Donor Kidney Transplantation.Medicine (Baltimore). 2016 Mar;95(11):e3076. doi: 10.1097/MD.0000000000003076. Medicine (Baltimore). 2016. PMID: 26986138 Free PMC article.
-
The role of novel biomarkers in early diagnosis and prognosis of acute kidney injury in newborns.Am J Perinatol. 2013 May;30(5):347-52. doi: 10.1055/s-0032-1326985. Epub 2012 Sep 21. Am J Perinatol. 2013. PMID: 23023560 Review.
Cited by
-
Postoperative anemia is a risk factor for acute kidney injury after open aorta and vena cava surgeries.PLoS One. 2020 Oct 13;15(10):e0240243. doi: 10.1371/journal.pone.0240243. eCollection 2020. PLoS One. 2020. PMID: 33048948 Free PMC article.
-
Erythropoiesis stimulating agents and reno-protection: a meta-analysis.BMC Nephrol. 2017 Jan 11;18(1):14. doi: 10.1186/s12882-017-0438-4. BMC Nephrol. 2017. PMID: 28077085 Free PMC article. Review.
-
Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis.Cochrane Database Syst Rev. 2023 Feb 13;2(2):CD010590. doi: 10.1002/14651858.CD010590.pub3. Cochrane Database Syst Rev. 2023. PMID: 36791280 Free PMC article.
-
Bridging Translation by Improving Preclinical Study Design in AKI.J Am Soc Nephrol. 2015 Dec;26(12):2905-16. doi: 10.1681/ASN.2015070832. Epub 2015 Nov 4. J Am Soc Nephrol. 2015. PMID: 26538634 Free PMC article. Review.
-
Co-treatment with Esculin and erythropoietin protects against renal ischemia-reperfusion injury via P2X7 receptor inhibition and PI3K/Akt activation.Sci Rep. 2022 Apr 14;12(1):6239. doi: 10.1038/s41598-022-09970-8. Sci Rep. 2022. PMID: 35422072 Free PMC article.
References
-
- NHS Blood and Transplant Annual Report. 2012. http://www.odt.nhs.uk/uk-transplant-registry/annual-activity-report.
-
- Leichtman AB, Cohen D, Keith D, O’Connor K, Goldstein M, McBride V, et al. Kidney and pancreas transplantation in the United States, 1997-2006: the HRSA Breakthrough Collaboratives and the 58 DSA Challenge. Am J Transplant. 2008;8(4 Pt 2):946–57. doi: 10.1111/j.1600-6143.2008.02173.x. - DOI - PubMed
-
- Moreno F, Sanz-Guajardo D, López-Gómez JM, Jofre R, Valderrábano F. Increasing the hematocrit has a beneficial effect on quality of life and is safe in selected hemodialysis patients. Spanish Cooperative Renal Patients Quality of Life Study Group of the Spanish Society of Nephrology. J Am Soc Nephrol. 2000;11:335–42. - PubMed
-
- Bárány P, Pettersson E, Bergström J. Erythropoietin treatment improves quality of life in hemodialysis patients. Scand J Urol Nephrol Suppl. 1990;131:55–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

