Aggregatibacter actinomycetemcomitans cytolethal distending toxin activates the NLRP3 inflammasome in human macrophages, leading to the release of proinflammatory cytokines
- PMID: 25644004
- PMCID: PMC4363449
- DOI: 10.1128/IAI.03132-14
Aggregatibacter actinomycetemcomitans cytolethal distending toxin activates the NLRP3 inflammasome in human macrophages, leading to the release of proinflammatory cytokines
Abstract
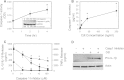
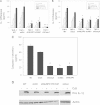
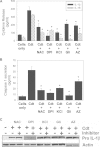
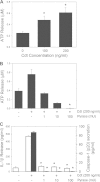
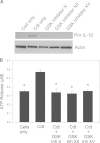
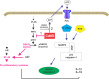
The cytolethal distending toxin (Cdt) is produced from a number of bacteria capable of causing infection and inflammatory disease. Our previous studies with Actinobacillus actinomycetemcomitans Cdt demonstrate not only that the active toxin subunit functions as a phosphatidylinositol-3,4,5-triphosphate (PIP3) phosphatase but also that macrophages exposed to the toxin were stimulated to produce proinflammatory cytokines. We now demonstrate that the Cdt-induced proinflammatory response involves the activation of the NLRP3 inflammasome. Specific inhibitors and short hairpin RNA (shRNA) were employed to demonstrate requirements for NLRP3 and ASC as well as caspase-1. Furthermore, Cdt-mediated inflammasome activation is dependent upon upstream signals, including reactive oxygen species (ROS) generation and Cdt-induced increases in extracellular ATP levels. Increases in extracellular ATP levels contribute to the activation of the P2X7 purinergic receptor, leading to K+ efflux. The relationship between the abilities of the active toxin subunit CdtB to function as a lipid phosphatase, activate the NLRP3 inflammasome, and induce a proinflammatory cytokine response is discussed. These studies provide new insight into the virulence potential of Cdt in mediating the pathogenesis of disease caused by Cdt-producing organisms such as Aggregatibacter actinomycetemcomitans.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Okuda J, Kurazono H, Takeda Y. 1995. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog 18:167–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

