Protein A is released into the Staphylococcus aureus culture supernatant with an unprocessed sorting signal
- PMID: 25644005
- PMCID: PMC4363444
- DOI: 10.1128/IAI.03122-14
Protein A is released into the Staphylococcus aureus culture supernatant with an unprocessed sorting signal
Abstract
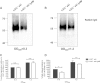
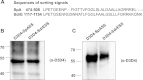
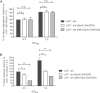
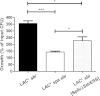
The immunoglobulin binding protein A (SpA) of Staphylococcus aureus is synthesized as a precursor with a C-terminal sorting signal. The sortase A enzyme mediates covalent attachment to peptidoglycan so that SpA is displayed on the surface of the bacterium. Protein A is also found in the extracellular medium, but the processes involved in its release are not fully understood. Here, we show that a portion of SpA is released into the supernatant with an intact sorting signal, indicating that it has not been processed by sortase A. Release of SpA was reduced when the native sorting signal of SpA was replaced with the corresponding region of another sortase-anchored protein (SdrE). Similarly, a reporter protein fused to the sorting signal of SpA was released to a greater extent than the same polypeptide fused to the SdrE sorting signal. Released SpA protected bacteria from killing in human blood, indicating that it contributes to immune evasion.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Staphylococcus epidermidis ΔSortase A strain elicits protective immunity against Staphylococcus aureus infection.Antonie Van Leeuwenhoek. 2017 Jan;110(1):133-143. doi: 10.1007/s10482-016-0784-4. Epub 2016 Oct 18. Antonie Van Leeuwenhoek. 2017. PMID: 27757703
-
Sortases, Surface Proteins, and Their Roles in Staphylococcus aureus Disease and Vaccine Development.Microbiol Spectr. 2019 Jan;7(1):10.1128/microbiolspec.psib-0004-2018. doi: 10.1128/microbiolspec.PSIB-0004-2018. Microbiol Spectr. 2019. PMID: 30737913 Free PMC article.
-
Molecular basis of surface anchored protein A deficiency in the Staphylococcus aureus strain Wood 46.PLoS One. 2017 Aug 31;12(8):e0183913. doi: 10.1371/journal.pone.0183913. eCollection 2017. PLoS One. 2017. PMID: 28859130 Free PMC article.
-
Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus.Mol Microbiol. 2001 Jun;40(5):1049-57. doi: 10.1046/j.1365-2958.2001.02411.x. Mol Microbiol. 2001. PMID: 11401711 Review.
-
Sortase-mediated ligation: a gift from Gram-positive bacteria to protein engineering.Chembiochem. 2009 Mar 23;10(5):787-98. doi: 10.1002/cbic.200800724. Chembiochem. 2009. PMID: 19199328 Review. No abstract available.
Cited by
-
Absence of Protein A Expression Is Associated With Higher Capsule Production in Staphylococcal Isolates.Front Microbiol. 2019 May 10;10:863. doi: 10.3389/fmicb.2019.00863. eCollection 2019. Front Microbiol. 2019. PMID: 31133995 Free PMC article.
-
Staphylococcus aureus foldase PrsA contributes to the folding and secretion of protein A.BMC Microbiol. 2024 Apr 2;24(1):108. doi: 10.1186/s12866-024-03268-7. BMC Microbiol. 2024. PMID: 38566014 Free PMC article.
-
The skin microbiome stratifies patients with cutaneous T cell lymphoma and determines event-free survival.NPJ Biofilms Microbiomes. 2024 Aug 29;10(1):74. doi: 10.1038/s41522-024-00542-4. NPJ Biofilms Microbiomes. 2024. PMID: 39198450 Free PMC article.
-
Manipulation of Innate and Adaptive Immunity by Staphylococcal Superantigens.Pathogens. 2018 May 29;7(2):53. doi: 10.3390/pathogens7020053. Pathogens. 2018. PMID: 29843476 Free PMC article. Review.
-
Tailor-made gene silencing of Staphylococcus aureus clinical isolates by CRISPR interference.PLoS One. 2018 Jan 29;13(1):e0185987. doi: 10.1371/journal.pone.0185987. eCollection 2018. PLoS One. 2018. PMID: 29377933 Free PMC article.
References
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core surveillance (ABCs) MRSA investigators . 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi:10.1001/jama.298.15.1763. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

