Developmental Programming of Branching Morphogenesis in the Kidney
- PMID: 25644110
- PMCID: PMC4587701
- DOI: 10.1681/ASN.2014090886
Developmental Programming of Branching Morphogenesis in the Kidney
Abstract
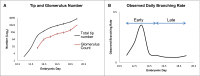
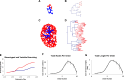
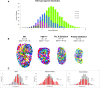
The kidney developmental program encodes the intricate branching and organization of approximately 1 million functional units (nephrons). Branching regulation is poorly understood, as is the source of a 10-fold variation in nephron number. Notably, low nephron count increases the risk for developing hypertension and renal failure. To better understand the source of this variation, we analyzed the complete gestational trajectory of mouse kidney development. We constructed a computerized architectural map of the branching process throughout fetal life and found that organogenesis is composed of two distinct developmental phases, each with stage-specific rate and morphologic parameters. The early phase is characterized by a rapid acceleration in branching rate and by branching divisions that repeat with relatively reproducible morphology. The latter phase, however, is notable for a significantly decreased yet constant branching rate and the presence of nonstereotyped branching events that generate progressive variability in tree morphology until birth. Our map identifies and quantitates the contribution of four developmental mechanisms that guide organogenesis: growth, patterning, branching rate, and nephron induction. When applied to organs that developed under conditions of malnutrition or in the setting of growth factor mutation, our normative map provided an essential link between kidney architecture and the fundamental morphogenetic mechanisms that guide development. This morphogenetic map is expected to find widespread applications and help identify modifiable targets to prevent developmental programming of common diseases.
Keywords: branching morphogenesis; kidney development; nephron number; variability.
Copyright © 2015 by the American Society of Nephrology.
Figures

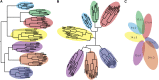
References
-
- Luyckx VA, Bertram JF, Brenner BM, Fall C, Hoy WE, Ozanne SE, Vikse BE: Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 382: 273–283, 2013 - PubMed
-
- Bagby SP: Developmental origins of renal disease: Should nephron protection begin at birth? Clin J Am Soc Nephrol 4: 10–13, 2009 - PubMed
-
- Brenner BM, Garcia DL, Anderson S: Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1: 335–347, 1988 - PubMed
-
- Puelles VG, Hoy WE, Hughson MD, Diouf B, Douglas-Denton RN, Bertram JF: Glomerular number and size variability and risk for kidney disease. Curr Opin Nephrol Hypertens 20: 7–15, 2011 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

