Neutrophil Extracellular Trap-Related Extracellular Histones Cause Vascular Necrosis in Severe GN
- PMID: 25644111
- PMCID: PMC4587690
- DOI: 10.1681/ASN.2014070673
Neutrophil Extracellular Trap-Related Extracellular Histones Cause Vascular Necrosis in Severe GN
Abstract
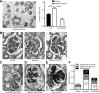
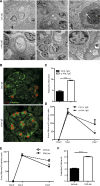
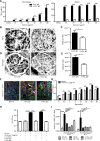
Severe GN involves local neutrophil extracellular trap (NET) formation. We hypothesized a local cytotoxic effect of NET-related histone release in necrotizing GN. In vitro, histones from calf thymus or histones released by neutrophils undergoing NETosis killed glomerular endothelial cells, podocytes, and parietal epithelial cells in a dose-dependent manner. Histone-neutralizing agents such as antihistone IgG, activated protein C, or heparin prevented this effect. Histone toxicity on glomeruli ex vivo was Toll-like receptor 2/4 dependent, and lack of TLR2/4 attenuated histone-induced renal thrombotic microangiopathy and glomerular necrosis in mice. Anti-glomerular basement membrane GN involved NET formation and vascular necrosis, whereas blocking NET formation by peptidylarginine inhibition or preemptive anti-histone IgG injection significantly reduced all aspects of GN (i.e., vascular necrosis, podocyte loss, albuminuria, cytokine induction, recruitment or activation of glomerular leukocytes, and glomerular crescent formation). To evaluate histones as a therapeutic target, mice with established GN were treated with three different histone-neutralizing agents. Anti-histone IgG, recombinant activated protein C, and heparin were equally effective in abrogating severe GN, whereas combination therapy had no additive effects. Together, these results indicate that NET-related histone release during GN elicits cytotoxic and immunostimulatory effects. Furthermore, neutralizing extracellular histones is still therapeutic when initiated in established GN.
Keywords: ARF; glomerular endothelial cells; immunology; pathology.
Copyright © 2015 by the American Society of Nephrology.
Figures





References
-
- Couser WG: Basic and translational concepts of immune-mediated glomerular diseases. J Am Soc Nephrol 23: 381–399, 2012 - PubMed
-
- Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 - PubMed
-
- Jennette JC, Xiao H, Falk RJ: Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol 17: 1235–1242, 2006 - PubMed
-
- Ryu M, Migliorini A, Miosge N, Gross O, Shankland S, Brinkkoetter PT, Hagmann H, Romagnani P, Liapis H, Anders HJ: Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury. J Pathol 228: 482–494, 2012 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

