Hypoxia-induced MTA1 promotes MC3T3 osteoblast growth but suppresses MC3T3 osteoblast differentiation
- PMID: 25644400
- PMCID: PMC4324858
- DOI: 10.1186/s40001-015-0084-x
Hypoxia-induced MTA1 promotes MC3T3 osteoblast growth but suppresses MC3T3 osteoblast differentiation
Abstract
Background: Bone fracture is one of the most common physical injuries in which gene expression and the microenvironment are reprogramed to facilitate the recovery process.
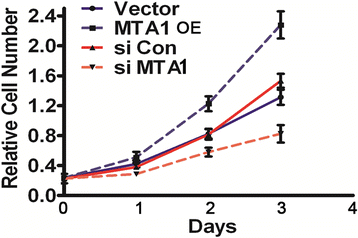
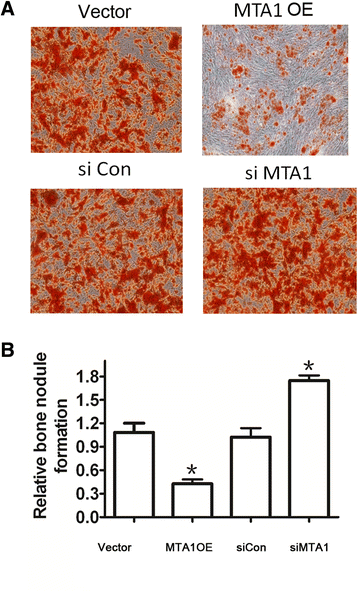
Methods: By specific siRNA transfection and MTT assay, we evaluated the effects of metastasis-associated gene 1 (MTA1) in osteoblast growth. To show the role of MTA1 in osteoblast under hypoxia conditions, by overexpressing and silencing MTA1 expression, we performed mineral deposition and alkaline phosphatase activity assay to observe the differentiation status of osteoblast cells. Real-time PCR and Western blot assays were adopted to detect the expression of certain target genes.
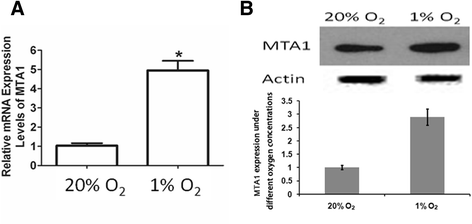
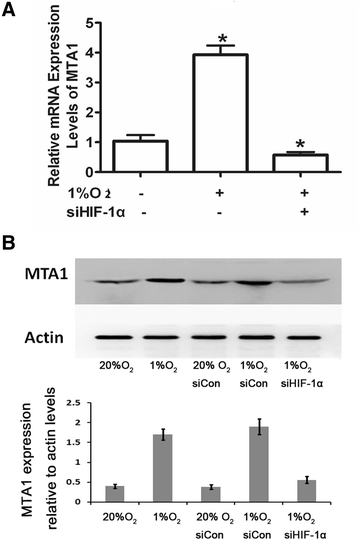
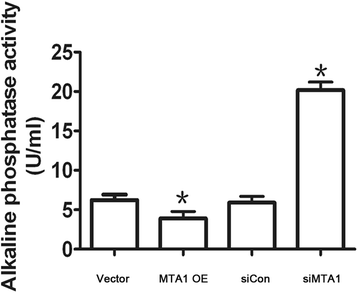
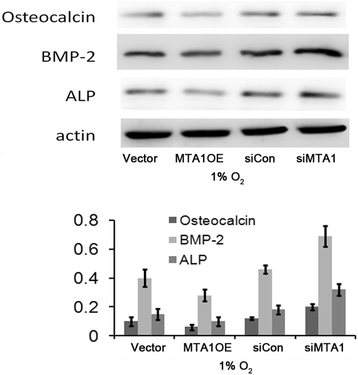
Results: Here, we reported that hypoxia-induced MTA1 expression through hypoxia-induced factor 1 alpha (HIF-1α) and stimulated the growth of osteoblast MC3T3 cells. Silencing of MTA1 through specific siRNA suppressed MC3T3 cell growth and elicited cell differentiation and induced alkaline phosphatase activation and the upregulation of bone morphogenetic protein-2 and osteocalcin.
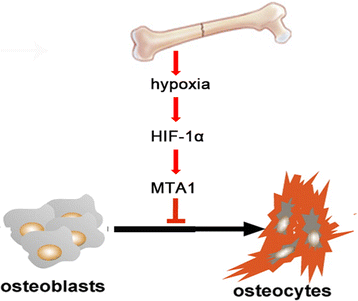
Conclusions: We found that MTA1 was regulated by HIF-1α in hypoxia circumstance to suppress osteoblast differentiation. These findings provide new insights for bone fracture healing and new strategies to develop potential targets to promote fracture healing.
Figures
Similar articles
-
Osteoblast-specific transcription factor Osterix (Osx) and HIF-1α cooperatively regulate gene expression of vascular endothelial growth factor (VEGF).Biochem Biophys Res Commun. 2012 Jul 20;424(1):176-81. doi: 10.1016/j.bbrc.2012.06.104. Epub 2012 Jun 27. Biochem Biophys Res Commun. 2012. PMID: 22750006
-
Reduction of protein phosphatase 2A Cα enhances bone formation and osteoblast differentiation through the expression of bone-specific transcription factor Osterix.Bone. 2011 Sep;49(3):368-75. doi: 10.1016/j.bone.2011.06.004. Epub 2011 Jun 12. Bone. 2011. PMID: 21683816
-
Synergistic inhibition of Wnt pathway by HIF-1α and osteoblast-specific transcription factor osterix (Osx) in osteoblasts.PLoS One. 2012;7(12):e52948. doi: 10.1371/journal.pone.0052948. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300831 Free PMC article.
-
[Regulation of hypoxia inducible factor-1alpha on osteoblast function in osteogenesis].Zhonghua Yi Xue Za Zhi. 2007 Dec 18;87(47):3357-61. Zhonghua Yi Xue Za Zhi. 2007. PMID: 18478952 Chinese.
-
Hypoxic downregulation of cellular proliferation and loss of phenotype stability in human osteoblasts is mediated by HIF-1α.Clin Hemorheol Microcirc. 2011;49(1-4):279-86. doi: 10.3233/CH-2011-1478. Clin Hemorheol Microcirc. 2011. PMID: 22214699
Cited by
-
Hypoxia Pathway in Osteoporosis: Laboratory Data for Clinical Prospects.Int J Environ Res Public Health. 2023 Feb 10;20(4):3129. doi: 10.3390/ijerph20043129. Int J Environ Res Public Health. 2023. PMID: 36833823 Free PMC article. Review.
-
Short-wave enhances mesenchymal stem cell recruitment in fracture healing by increasing HIF-1 in callus.Stem Cell Res Ther. 2020 Sep 7;11(1):382. doi: 10.1186/s13287-020-01888-0. Stem Cell Res Ther. 2020. PMID: 32894200 Free PMC article.
-
Effects of normobaric cyclic hypoxia exposure on mesenchymal stem-cell differentiation-pilot study on bone parameters in elderly.World J Stem Cells. 2020 Dec 26;12(12):1667-1690. doi: 10.4252/wjsc.v12.i12.1667. World J Stem Cells. 2020. PMID: 33505607 Free PMC article.
-
CRISPR/Cas9 knockout of MTA1 enhanced RANKL-induced osteoclastogenesis in RAW264.7 cells partly via increasing ROS activities.J Cell Mol Med. 2023 Mar;27(5):701-713. doi: 10.1111/jcmm.17692. Epub 2023 Feb 14. J Cell Mol Med. 2023. PMID: 36786127 Free PMC article.
-
Correlation of two distinct metastasis-associated proteins, MTA1 and S100A4, in angiogenesis for promoting tumor growth.Oncogene. 2019 Jun;38(24):4715-4728. doi: 10.1038/s41388-019-0748-z. Epub 2019 Feb 11. Oncogene. 2019. PMID: 30745574
References
-
- Heppenstall RB, Goodwin CW, Brighton CT. Fracture healing in the presence of chronic hypoxia. J Bone Joint Surg Am. 1976;58:1153–6. - PubMed
-
- Akeno N, Czyzyk-Krzeska MF, Gross TS, Clemens TL. Hypoxia induces vascular endothelial growth factor gene transcription in human osteoblast-like cells through the hypoxia-inducible factor-2alpha. Endocrinology. 2001;142:959–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

