Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells
- PMID: 25644533
- PMCID: PMC4409767
- DOI: 10.1016/j.yjmcc.2015.01.013
Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells
Abstract
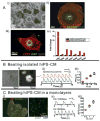
Background and objectives: The creation of cardiomyocytes derived from human induced pluripotent stem cells (hiPS-CMs) has spawned broad excitement borne out of the prospects to diagnose and treat cardiovascular diseases based on personalized medicine. A common feature of hiPS-CMs is their spontaneous contractions but the mechanism(s) remain uncertain.
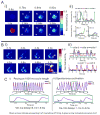
Methods: Intrinsic activity was investigated by the voltage-clamp technique, optical mapping of action potentials (APs) and intracellular Ca(2+) (Cai) transients (CaiT) at subcellular-resolution and pharmacological interventions.
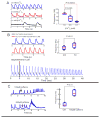
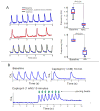
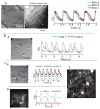
Results: The frequency of spontaneous CaiT (sCaiT) in monolayers of hiPS-CMs was not altered by ivabradine, an inhibitor of the pacemaker current, If despite high levels of HCN transcripts (1-4). HiPS-CMs had negligible If and IK1 (inwardly-rectifying K(+)-current) and a minimum diastolic potential of -59.1±3.3mV (n=18). APs upstrokes were preceded by a depolarizing-foot coincident with a rise of Cai. Subcellular Cai wavelets varied in amplitude, propagated and died-off; larger Cai-waves triggered cellular sCaTs and APs. SCaiTs increased in frequency with [Ca(2+)]out (0.05-to-1.8mM), isoproterenol (1μM) or caffeine (100μM) (n≥5, p<0.05). HiPS-CMs became quiescent with ryanodine receptor stabilizers (K201=2μM); tetracaine; Na-Ca exchange (NCX) inhibition (SEA0400=2μM); higher [K(+)]out (5→8mM), and thiol-reducing agents but could still be electrically stimulated to elicit CaiTs. Cell-cell coupling of hiPS-CM in monolayers was evident from connexin-43 expression and CaiT propagation. SCaiTs from an ensemble of dispersed hiPS-CMs were out-of-phase but became synchronous through the outgrowth of inter-connecting microtubules.
Conclusions: Automaticity in hiPS-CMs originates from a Ca(2+)-clock mechanism involving Ca(2+) cycling across the sarcoplasmic reticulum linked to NCX to trigger APs.
Keywords: Cell–cell coupling; Funny current; Human myocytes from stem cells; Optical mapping of calcium and action potentials; Spontaneous activity; Subcellular calcium waves.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflicts to declare
Figures


References
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. - PubMed
-
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. - PubMed
-
- Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, et al. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24:236–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000005/TR/NCATS NIH HHS/United States
- R01 HL057929/HL/NHLBI NIH HHS/United States
- HL062465/HL/NHLBI NIH HHS/United States
- HL093631/HL/NHLBI NIH HHS/United States
- HL70722/HL/NHLBI NIH HHS/United States
- DP1OD003819/OD/NIH HHS/United States
- R01 HL070722/HL/NHLBI NIH HHS/United States
- R01 HL069097/HL/NHLBI NIH HHS/United States
- R21 HL093631/HL/NHLBI NIH HHS/United States
- DP2 HL127727/HL/NHLBI NIH HHS/United States
- HL093074/HL/NHLBI NIH HHS/United States
- R01 HL093074/HL/NHLBI NIH HHS/United States
- R01 HL062465/HL/NHLBI NIH HHS/United States
- R01 HL059614/HL/NHLBI NIH HHS/United States
- DP1 OD003819/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

