Lamins at the crossroads of mechanosignaling
- PMID: 25644599
- PMCID: PMC4318140
- DOI: 10.1101/gad.255968.114
Lamins at the crossroads of mechanosignaling
Abstract
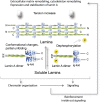
The intermediate filament proteins, A- and B-type lamins, form the nuclear lamina scaffold adjacent to the inner nuclear membrane. B-type lamins confer elasticity, while A-type lamins lend viscosity and stiffness to nuclei. Lamins also contribute to chromatin regulation and various signaling pathways affecting gene expression. The mechanical roles of lamins and their functions in gene regulation are often viewed as independent activities, but recent findings suggest a highly cross-linked and interdependent regulation of these different functions, particularly in mechanosignaling. In this newly emerging concept, lamins act as a "mechanostat" that senses forces from outside and responds to tension by reinforcing the cytoskeleton and the extracellular matrix. A-type lamins, emerin, and the linker of the nucleoskeleton and cytoskeleton (LINC) complex directly transmit forces from the extracellular matrix into the nucleus. These mechanical forces lead to changes in the molecular structure, modification, and assembly state of A-type lamins. This in turn activates a tension-induced "inside-out signaling" through which the nucleus feeds back to the cytoskeleton and the extracellular matrix to balance outside and inside forces. These functions regulate differentiation and may be impaired in lamin-linked diseases, leading to cellular phenotypes, particularly in mechanical load-bearing tissues.
Keywords: LINC complex; cytoskeleton; extracellular matrix; lamins; mechanosensing; mechanotransduction.
© 2015 Osmanagic-Myers et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Aebi U, Cohn J, Buhle L, Gerace L. 1986. The nuclear lamina is a meshwork of intermediate-type filaments. Nature 323: 560–564. - PubMed
-
- Bank EM, Ben-Harush K, Feinstein N, Medalia O, Gruenbaum Y. 2012. Structural and physiological phenotypes of disease-linked lamin mutations in C. elegans. J Struct Biol 177: 106–112. - PubMed
-
- Beavan LA, Quentin-Hoffmann E, Schonherr E, Snigula F, Leroy JG, Kresse H. 1993. Deficient expression of decorin in infantile progeroid patients. J Biol Chem 268: 9856–9862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
