An evolutionarily conserved switch in response to GABA affects development and behavior of the locomotor circuit of Caenorhabditis elegans
- PMID: 25644702
- PMCID: PMC4391577
- DOI: 10.1534/genetics.114.173963
An evolutionarily conserved switch in response to GABA affects development and behavior of the locomotor circuit of Caenorhabditis elegans
Abstract
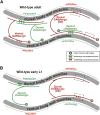
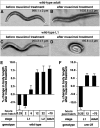
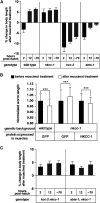
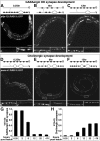
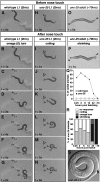
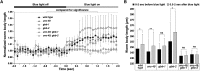
The neurotransmitter gamma-aminobutyric acid (GABA) is depolarizing in the developing vertebrate brain, but in older animals switches to hyperpolarizing and becomes the major inhibitory neurotransmitter in adults. We discovered a similar developmental switch in GABA response in Caenorhabditis elegans and have genetically analyzed its mechanism and function in a well-defined circuit. Worm GABA neurons innervate body wall muscles to control locomotion. Activation of GABAA receptors with their agonist muscimol in newly hatched first larval (L1) stage animals excites muscle contraction and thus is depolarizing. At the mid-L1 stage, as the GABAergic neurons rewire onto their mature muscle targets, muscimol shifts to relaxing muscles and thus has switched to hyperpolarizing. This muscimol response switch depends on chloride transporters in the muscles analogous to those that control GABA response in mammalian neurons: the chloride accumulator sodium-potassium-chloride-cotransporter-1 (NKCC-1) is required for the early depolarizing muscimol response, while the two chloride extruders potassium-chloride-cotransporter-2 (KCC-2) and anion-bicarbonate-transporter-1 (ABTS-1) are required for the later hyperpolarizing response. Using mutations that disrupt GABA signaling, we found that neural circuit development still proceeds to completion but with an ∼6-hr delay. Using optogenetic activation of GABAergic neurons, we found that endogenous GABAA signaling in early L1 animals, although presumably depolarizing, does not cause an excitatory response. Thus a developmental depolarizing-to-hyperpolarizing shift is an ancient conserved feature of GABA signaling, but existing theories for why this shift occurs appear inadequate to explain its function upon rigorous genetic analysis of a well-defined neural circuit.
Keywords: GABA response; inhibitory; signaling; switch.
Copyright © 2015 by the Genetics Society of America.
Figures

Similar articles
-
Two types of chloride transporters are required for GABA(A) receptor-mediated inhibition in C. elegans.EMBO J. 2011 May 4;30(9):1852-63. doi: 10.1038/emboj.2011.83. Epub 2011 Mar 22. EMBO J. 2011. PMID: 21427702 Free PMC article.
-
Depolarizing chloride gradient in developing cochlear nucleus neurons: underlying mechanism and implication for calcium signaling.Neuroscience. 2014 Mar 7;261:207-22. doi: 10.1016/j.neuroscience.2013.12.050. Epub 2014 Jan 3. Neuroscience. 2014. PMID: 24388924
-
Evidence for Two Subpopulations of Cerebrospinal Fluid-Contacting Neurons with Opposite GABAergic Signaling in Adult Mouse Spinal Cord.J Neurosci. 2024 May 29;44(22):e2289222024. doi: 10.1523/JNEUROSCI.2289-22.2024. J Neurosci. 2024. PMID: 38684364 Free PMC article.
-
Double-edged GABAergic synaptic transmission in seizures: The importance of chloride plasticity.Brain Res. 2018 Dec 15;1701:126-136. doi: 10.1016/j.brainres.2018.09.008. Epub 2018 Sep 7. Brain Res. 2018. PMID: 30201259 Review.
-
GABA neurotransmission and neural cation-chloride co-transporters: actions beyond ion transport.Crit Rev Neurobiol. 2006;18(1-2):105-12. doi: 10.1615/critrevneurobiol.v18.i1-2.110. Crit Rev Neurobiol. 2006. PMID: 17725513 Review.
Cited by
-
Transcranial Magnetic Stimulation in Tourette Syndrome: A Historical Perspective, Its Current Use and the Influence of Comorbidities in Treatment Response.Brain Sci. 2018 Jul 6;8(7):129. doi: 10.3390/brainsci8070129. Brain Sci. 2018. PMID: 29986411 Free PMC article. Review.
-
An Optogenetic Approach for Investigation of Excitatory and Inhibitory Network GABA Actions in Mice Expressing Channelrhodopsin-2 in GABAergic Neurons.J Neurosci. 2016 Jun 1;36(22):5961-73. doi: 10.1523/JNEUROSCI.3482-15.2016. J Neurosci. 2016. PMID: 27251618 Free PMC article.
-
A Change in the Ion Selectivity of Ligand-Gated Ion Channels Provides a Mechanism to Switch Behavior.PLoS Biol. 2015 Sep 8;13(9):e1002238. doi: 10.1371/journal.pbio.1002238. eCollection 2015. PLoS Biol. 2015. PMID: 26348462 Free PMC article.
-
Combining single-cell RNA-sequencing with a molecular atlas unveils new markers for Caenorhabditis elegans neuron classes.Nucleic Acids Res. 2020 Jul 27;48(13):7119-7134. doi: 10.1093/nar/gkaa486. Nucleic Acids Res. 2020. PMID: 32542321 Free PMC article.
-
The transforming growth factor beta ligand TIG-2 modulates the function of neuromuscular junction and muscle energy metabolism in Caenorhabditis elegans.Front Mol Neurosci. 2022 Oct 28;15:962974. doi: 10.3389/fnmol.2022.962974. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36385772 Free PMC article.
References
-
- Beg A. A., Jorgensen E. M., 2003. EXP-1 is an excitatory GABA-gated cation channel. Nat. Neurosci. 6: 1145–1152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

