Anti-inflammatory activity of Wnt signaling in enteric nervous system: in vitro preliminary evidences in rat primary cultures
- PMID: 25644719
- PMCID: PMC4332439
- DOI: 10.1186/s12974-015-0248-1
Anti-inflammatory activity of Wnt signaling in enteric nervous system: in vitro preliminary evidences in rat primary cultures
Abstract
Background: In the last years, Wnt signaling was demonstrated to regulate inflammatory processes. In particular, an increased expression of Wnts and Frizzled receptors was reported in inflammatory bowel disease (IBD) and ulcerative colitis to exert both anti- and pro-inflammatory functions regulating the intestinal activated nuclear factor κB (NF-кB), TNFa release, and IL10 expression.
Methods: To investigate the role of Wnt pathway in the response of the enteric nervous system (ENS) to inflammation, neurons and glial cells from rat myenteric plexus were treated with exogenous Wnt3a and/or LPS with or without supporting neurotrophic factors such as basic fibroblast growth factor (bFGF), epithelial growth factor (EGF), and glial cell-derived neurotrophic factor (GDNF). The immunophenotypical characterization by flow cytometry and the protein and gene expression analysis by qPCR and Western blotting were carried out.
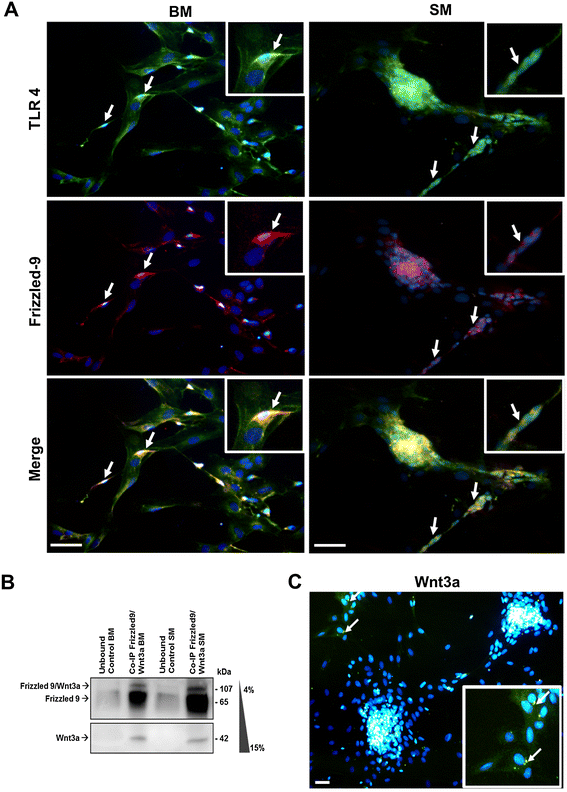
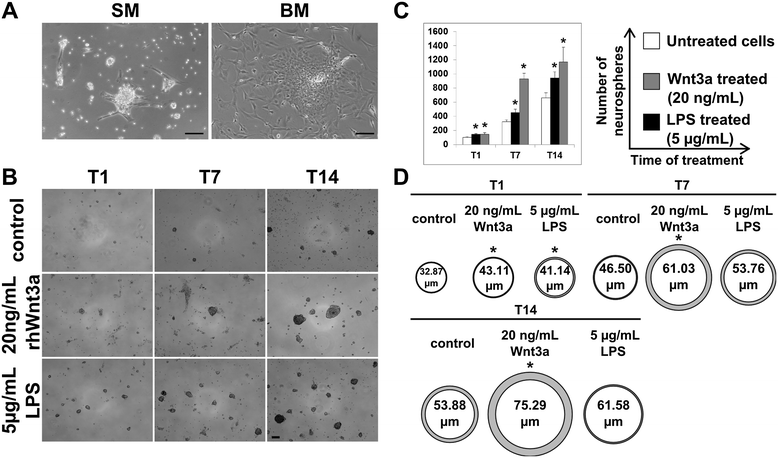
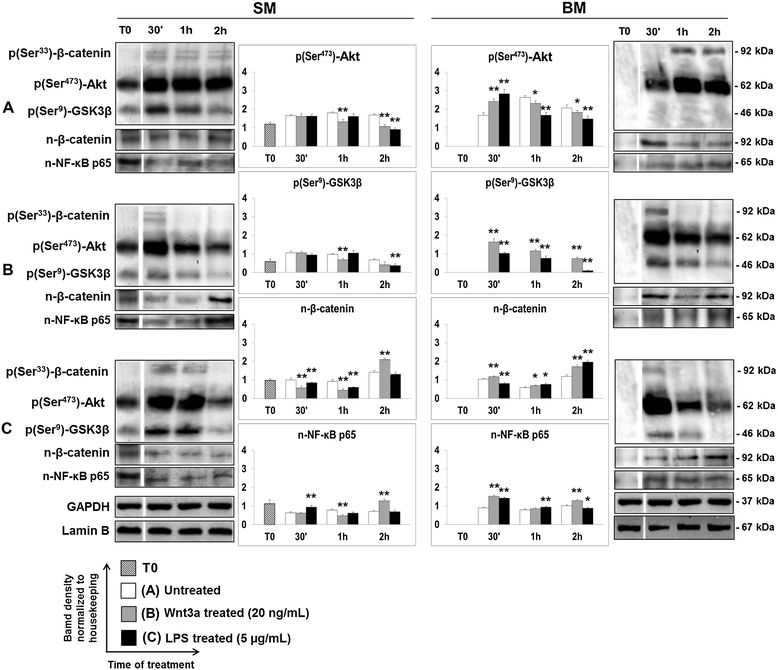
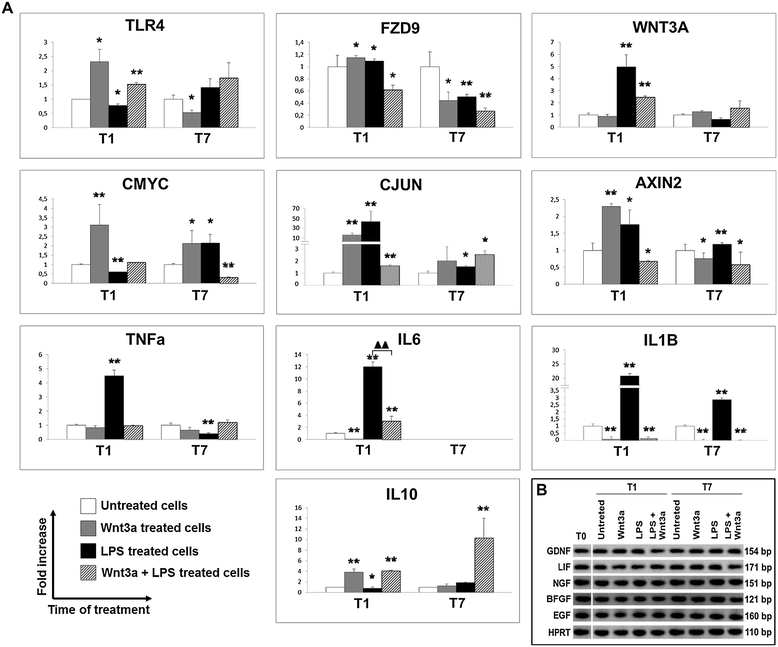
Results: Flow cytometry and immunofluorescence staining evidenced that enteric neurons coexpressed Frizzled 9 and toll-like receptor 4 (TLR4) while glial cells were immunoreactive to TLR4 and Wnt3a suggesting that canonical Wnt signaling is active in ENS. Under in vitro LPS treatment, Western blot analysis demonstrated an active cross talk between canonical Wnt signaling and NF-кB pathway that is essential to negatively control enteric neuronal response to inflammatory stimuli. Upon costimulation with LPS and Wnt3a, a significant anti-inflammatory activity was detected by RT-PCR based on an increased IL10 expression and a downregulation of pro-inflammatory cytokines TNFa, IL1B, and interleukin 6 (IL6). When the availability of neurotrophic factors in ENS cultures was abolished, a changed cell reactivity by Wnt signaling was observed at basal conditions and after LPS treatment.
Conclusions: The results of this study suggested the existence of neuronal surveillance through FZD9 and Wnt3a in enteric myenteric plexus. Moreover, experimental evidences were provided to clarify the correlation among soluble trophic factors, Wnt signaling, and anti-inflammatory protection of ENS.
Figures



References
-
- Gregorieff A, Pinto D, Begthel H, Destrée O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129(2):626–38. - PubMed
-
- Wodarz A, Nusse R. Mechanisms of Wnt signalling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. - PubMed
-
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, et al. A new member of the frizzled family from Drosophila functions as a wingless receptor. Nature. 1996;382(6588):225–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

