The Effect of Cadmium on COX-1 and COX-2 Gene, Protein Expression, and Enzymatic Activity in THP-1 Macrophages
- PMID: 25645360
- PMCID: PMC4424267
- DOI: 10.1007/s12011-015-0234-6
The Effect of Cadmium on COX-1 and COX-2 Gene, Protein Expression, and Enzymatic Activity in THP-1 Macrophages
Abstract
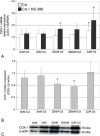
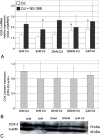
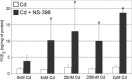
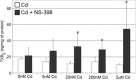
The aim of this study was to examine the effects of cadmium in concentrations relevant to those detected in human serum on cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) expression at mRNA, protein, and enzyme activity levels in THP-1 macrophages. Macrophages were incubated with various cadmium chloride (CdCl2) solutions for 48 h at final concentrations of 5 nM, 20 nM, 200 nM, and 2 μM CdCl2. The mRNA expression and protein levels of COXs were analyzed with RT-PCR and Western blotting, respectively. Prostaglandin E2 (PGE2) and stable metabolite of thromboxane B2 (TXB2) concentrations in culture media were determined using ELISA method. Our study demonstrates that cadmium at the highest tested concentrations modulates COX-1 and COX-2 at mRNA level in THP-1 macrophages; however, the lower tested cadmium concentrations appear to inhibit COX-1 protein expression. PGE2 and TXB2 production is not altered by all tested Cd concentrations; however, the significant stimulation of PGE2 and TXB2 production is observed when macrophages are exposed to both cadmium and COX-2 selective inhibitor, NS-398. The stimulatory effect of cadmium on COXs at mRNA level is not reflected at protein and enzymatic activity levels, suggesting the existence of some posttranscriptional, translational, and posttranslational events that result in silencing of those genes' expression.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

