Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions
- PMID: 25645576
- PMCID: PMC4327973
- DOI: 10.1186/s13018-014-0144-x
Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions
Abstract
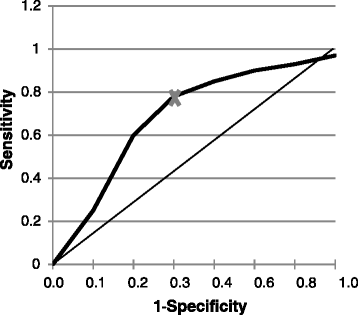
The overarching goals of treatments for orthopedic conditions are generally to improve or restore function and alleviate pain. Results of clinical trials are generally used to determine whether a treatment is efficacious; however, a statistically significant improvement may not actually be clinically important, i.e., meaningful to the patient. To determine whether an intervention has produced clinically important benefits requires a two-step process: first, determining the magnitude of change considered clinically important for a particular measure in the relevant population and, second, applying this yardstick to a patient's data to determine whether s/he has benefited from treatment. Several metrics have been devised to quantify clinically important differences, including the minimum clinically important difference (MCID) and clinically important difference (CID). Herein, we review the methods to generate the MCID and other metrics and their use and interpretation in clinical trials and practice. We particularly highlight the many pitfalls associated with the generation and utilization of these metrics that can impair their correct use. These pitfalls include the fact that different pain measures yield different MCIDs, that efficacy in clinical trials is impacted by various factors (population characteristics, trial design), that the MCID value is impacted by the method used to calculate it (anchor, distribution), by the type of anchor chosen and by the definition (threshold) of improvement. The MCID is also dependent on the population characteristics such as disease type and severity, sex, age, etc. For appropriate use, the MCID should be applied to changes in individual subjects, not to group changes. The MCID and CID are useful tools to define general guidelines to determine whether a treatment produces clinically meaningful effects. However, the many pitfalls associated with these metrics require a detailed understanding of the methods to calculate them and their context of use. Orthopedic surgeons that will use these metrics need to carefully understand them and be aware of their pitfalls.
Figures


References
-
- Pham T, Der Heijde DV, Lassere M, Altman RD, Anderson JJ, Bellamy N, et al. Outcome variables for osteoarthritis clinical trials: the OMERACT-OARSI set of responder criteria. J Rheumatol. 2003;30:1648–1654 - PubMed
-
- Morley S, Williams AC. Conducting and evaluating treatment outcome studies. In: Turk DC, Gatchel RJ, editors. Psychological approaches to pain management: a practitioner’s handbook. 2. New York: Guilford; 2002.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

