Quercetin sensitizes fluconazole-resistant candida albicans to induce apoptotic cell death by modulating quorum sensing
- PMID: 25645848
- PMCID: PMC4356769
- DOI: 10.1128/AAC.03599-14
Quercetin sensitizes fluconazole-resistant candida albicans to induce apoptotic cell death by modulating quorum sensing
Abstract
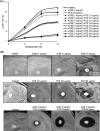
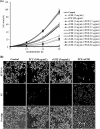
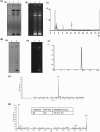
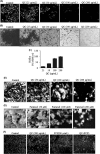
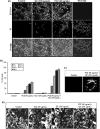
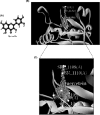
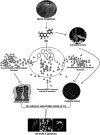
Quorum sensing (QS) regulates group behaviors of Candida albicans such as biofilm, hyphal growth, and virulence factors. The sesquiterpene alcohol farnesol, a QS molecule produced by C. albicans, is known to regulate the expression of virulence weapons of this fungus. Fluconazole (FCZ) is a broad-spectrum antifungal drug that is used for the treatment of C. albicans infections. While FCZ can be cytotoxic at high concentrations, our results show that at much lower concentrations, quercetin (QC), a dietary flavonoid isolated from an edible lichen (Usnea longissima), can be implemented as a sensitizing agent for FCZ-resistant C. albicans NBC099, enhancing the efficacy of FCZ. QC enhanced FCZ-mediated cell killing of NBC099 and also induced cell death. These experiments indicated that the combined application of both drugs was FCZ dose dependent rather than QC dose dependent. In addition, we found that QC strongly suppressed the production of virulence weapons-biofilm formation, hyphal development, phospholipase, proteinase, esterase, and hemolytic activity. Treatment with QC also increased FCZ-mediated cell death in NBC099 biofilms. Interestingly, we also found that QC enhances the anticandidal activity of FCZ by inducing apoptotic cell death. We have also established that this sensitization is reliant on the farnesol response generated by QC. Molecular docking studies also support this conclusion and suggest that QC can form hydrogen bonds with Gln969, Thr1105, Ser1108, Arg1109, Asn1110, and Gly1061 in the ATP binding pocket of adenylate cyclase. Thus, this QS-mediated combined sensitizer (QC)-anticandidal agent (FCZ) strategy may be a novel way to enhance the efficacy of FCZ-based therapy of C. albicans infections.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Castelo-Branco DS, Brilhante RS, Paiva MA, Teixeira CE, Caetano EP, Ribeiro JF, Cordeiro RA, Sidrim JJ, Monteiro AJ, Rocha MF. 2013. Azole-resistant Candida albicans from a wild Brazilian porcupine (Coendou prehensilis): a sign of an environmental imbalance? Med Mycol 51:555–560. doi:10.3109/13693786.2012.752878. - DOI - PubMed
-
- Youngsaye W, Hartland CL, Morgan BJ, Ting A, Nag PP, Vincent B, Mosher CA, Bittker JA, Dandapani S, Palmer M, Whitesell L, Lindquist S, Schreiber SL, Munoz B. 2013. ML212: A small-molecule probe for investigating fluconazole resistance mechanisms in Candida albicans. Beilstein J Org Chem 9:1501–1507. doi:10.3762/bjoc.9.171. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical

