Investigating the immunomodulatory nature of zinc oxide nanoparticles at sub-cytotoxic levels in vitro and after intranasal instillation in vivo
- PMID: 25645871
- PMCID: PMC4324663
- DOI: 10.1186/s12951-015-0067-7
Investigating the immunomodulatory nature of zinc oxide nanoparticles at sub-cytotoxic levels in vitro and after intranasal instillation in vivo
Abstract
Background: This study evaluates the time-dependent pro-inflammatory response of the model human lung epithelial cells (A549) to industrially relevant zinc oxide nanoparticles (ZnO NPs). In terms of toxicity, ZnO-NPs are categorised into the group of high toxicity nanomaterials. However information on pro-inflammatory potential of these NPs at sub-toxic concentrations is limited. Understanding how cellular defense mechanisms function in the presence of sub-cytotoxic concentrations of these NPs is vital. Moreover, there is an urgent need for additional in vivo studies addressing pulmonary toxicity due to accidental inhalation of ZnO NPs.
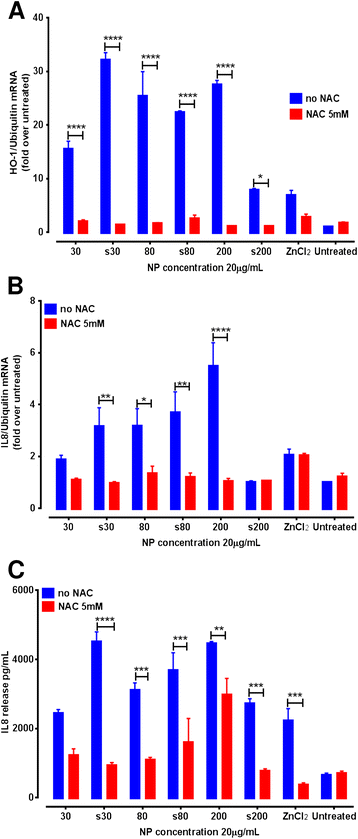
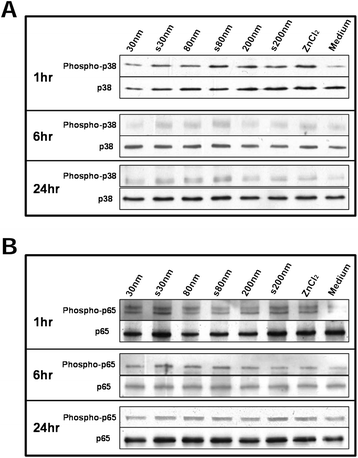
Results: Exposure to sub-cytotoxic ZnO NP concentrations (20 μg/mL) induced significant up-regulation of mRNA for the pro-inflammatory cytokine IL-8 and redox stress marker heme oxygenase-1, along with increased release of IL-8. The highest pro-inflammatory response was recorded after 4 to 6 hr exposure to ZnO NPs over a 24 hr period. Pre-treatment of A549 cells with the sulfhydryl antioxidant N-acetyl cysteine (at 5 mM) resulted in significant reduction of the up-regulation of inflammatory markers, confirming the role of reactive oxygen species in the observed immunomodulatory effects, independent of cytotoxicity. Furthermore, we report for the first time that, intranasal instillation of a single dose (5 mg/kg) of pristine or surfactant-dispersed ZnO NPs can cause pulmonary inflammation, already after 24 hr in a murine model. This was confirmed by up-regulation of eotaxin mRNA in the lung tissue and release of pro-inflammatory cytokines in the sera of mice exposed to ZnO NPs.
Conclusion: Our study highlights that even at sub-cytotoxic doses ZnO NPs can stimulate a strong inflammatory and antioxidant response in A549 cells. ZnO NP mediated cytotoxicity may be the outcome of failure of cellular redox machinery to contain excessive ROS formation. Moreover exposure to a single but relatively high dose of ZnO NPs via intranasal instillation may provoke acute pulmonary inflammatory reactions in vivo.
Figures




References
-
- Wang ZL. Zinc oxide nanostructures: growth, properties and applications. J Phys Condens Matter. 2004;16:R829–58. doi: 10.1088/0953-8984/16/25/R01. - DOI
-
- Lin W, Xu Y, Huang C-C, Ma Y, Shannon K, Chen D-R, et al. Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. J Nanoparticle Res. 2009;11:25–39. doi: 10.1007/s11051-008-9419-7. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

