The efficacy and safety of rituximab in treating childhood refractory nephrotic syndrome: a meta-analysis
- PMID: 25645999
- PMCID: PMC4314653
- DOI: 10.1038/srep08219
The efficacy and safety of rituximab in treating childhood refractory nephrotic syndrome: a meta-analysis
Abstract
Rituximab is considered to be a promising drug for treating childhood refractory nephrotic syndrome. However, the efficacy and safety of rituximab in treating childhood refractory nephrotic syndrome remain inconclusive. This meta-analysis aimed to investigate the efficacy and safety of rituximab treatment compared with other immunosuppressive agents in children with refractory nephrotic syndrome. Three randomized controlled trials and two comparative control studies were included in our analysis. The included studies were of moderately high quality. Compared with other immunotherapies, rituximab therapy significantly improved relapse-free survival (hazard ratio = 0.49, 95% confidence interval [CI], 0.26-0.92, P = 0.03). Rituximab also achieved a higher rate of complete remission (risk ratio,1.62; 95% CI, 0.92 to 2.84, P = 0.09) and reduced the occurrence of proteinuria (mean difference = -0.25, 95% CI = -0.29 to -0.21, P < 0.00001); however, a more targeted rituximab treatment did not significantly increase serum albumin levels and did not significantly reduce adverse events. Rituximab might be a promising treatment for childhood refractory nephrotic syndrome; however, the long-term effects and cost-effectiveness of rituximab treatment were not fully assessed, and there were limited studies that evaluated the clinical benefits of a concurrent infusion of rituximab plus a steroid compared with an infusion of rituximab only. Additional studies are required to address these issues.
Figures
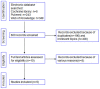



References
-
- Certikova-Chabova V. & Tesar V. Recent insights into the pathogenesis of nephrotic syndrome. Minerva Med 104, 333–347 (2013). - PubMed
-
- Eddy A. A. & Symons J. M. Nephrotic syndrome in childhood. Lancet 362, 629–639 (2003). - PubMed
-
- McKinney P. A., Feltbower R. G., Brocklebank J. T. & Fitzpatrick M. M. Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol 16, 1040–1044 (2001). - PubMed
-
- Tarshish P., Tobin J. N., Bernstein J. & Edelmann C. J. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8, 769–776 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

