Steroid differentiation: the safety profile of various steroids on retinal cells in vitro and their implications for clinical use (an American Ophthalmological Society thesis)
- PMID: 25646032
- PMCID: PMC4311675
Steroid differentiation: the safety profile of various steroids on retinal cells in vitro and their implications for clinical use (an American Ophthalmological Society thesis)
Abstract
Purpose: To determine if potentially viable alternatives to the clinical use of intravitreal triamcinolone acetonide should be considered based on a comparative assessment of the in vitro effects of five commercially available corticosteroids. We hypothesized that dexamethasone, betamethasone, methylprednisolone, loteprednol etabonate, and fluocinolone acetonide, at clinically relevant doses, may show different levels of in vitro cytotoxicity to retinal cells.
Methods: Cultures of human retinal pigment epithelial cells (ARPE-19) and rat embryonal neurosensory precursor retinal cells (R28) were treated with dexamethasone, betamethasone, methylprednisolone, loteprednol, or fluocinolone acetonide. Cell viability as a measure of cell death was determined by trypan blue dye exclusion assay. The mechanical effect of drug crystals was evaluated by solubilizing the steroid formulations. Mitochondrial dehydrogenase and membrane potential were assessed to measure cell damage.
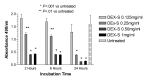
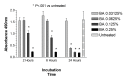
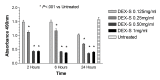
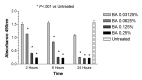
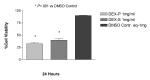
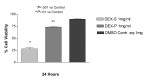
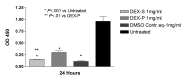
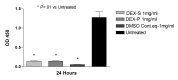
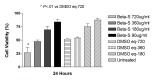
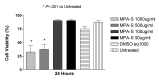
Results: Betamethasone, loteprednol, and methylprednisolone, in commercially available forms, caused significant cytotoxic changes to retinal cells in vitro at clinically relevant doses. This effect was less pronounced with solubilized betamethasone. Dexamethasone at concentrations up to 5 times the clinical dose of free drug injections and 1000 times greater than a drug implant did not cause decreased cell viability. Fluocinolone acetonide at doses 1000 times higher than observed with drug delivery systems showed no cytotoxic effect.
Conclusions: Betamethasone, loteprednol, and methylprednisolone exhibited cytotoxicity at clinically relevant doses and do not appear to be good therapeutic options for intravitreal use. In comparison, dexamethasone and fluocinolone acetonide, which exhibited fewer cytotoxic effects than other steroids, may be potentially viable alternatives to triamcinolone acetonide for clinical use.
Figures


















Similar articles
-
Toxicity of triamcinolone acetonide on retinal neurosensory and pigment epithelial cells.Invest Ophthalmol Vis Sci. 2006 Feb;47(2):722-8. doi: 10.1167/iovs.05-0772. Invest Ophthalmol Vis Sci. 2006. PMID: 16431973
-
Differential cytotoxicity of corticosteroids on human mesenchymal stem cells.Clin Orthop Relat Res. 2015 Mar;473(3):1155-64. doi: 10.1007/s11999-014-3925-y. Epub 2014 Sep 4. Clin Orthop Relat Res. 2015. PMID: 25187334 Free PMC article.
-
Trypan blue: effect on retinal pigment epithelial and neurosensory retinal cells.Invest Ophthalmol Vis Sci. 2005 Jan;46(1):304-9. doi: 10.1167/iovs.04-0703. Invest Ophthalmol Vis Sci. 2005. PMID: 15623789
-
Intravitreal steroids for macular edema in diabetes.Cochrane Database Syst Rev. 2020 Nov 17;11(11):CD005656. doi: 10.1002/14651858.CD005656.pub3. Cochrane Database Syst Rev. 2020. PMID: 33206392 Free PMC article.
-
Treatment of diabetic macular edema with sustained-release glucocorticoids: intravitreal triamcinolone acetonide, dexamethasone implant, and fluocinolone acetonide implant.Expert Opin Pharmacother. 2014 May;15(7):953-9. doi: 10.1517/14656566.2014.896899. Epub 2014 Mar 24. Expert Opin Pharmacother. 2014. PMID: 24661081 Review.
Cited by
-
Triamcinolone Acetonide-Assisted Visualization and Removal of Vitreous Cortex Remnants in Retinal Detachment: A Prospective Cohort Study.Diagnostics (Basel). 2025 Jul 23;15(15):1854. doi: 10.3390/diagnostics15151854. Diagnostics (Basel). 2025. PMID: 40804819 Free PMC article.
-
[Intramuscular depot steroids : Possible treatment of postsurgical cystoid macula edema with steroid response?].Ophthalmologe. 2017 Nov;114(11):1034-1037. doi: 10.1007/s00347-016-0425-3. Ophthalmologe. 2017. PMID: 28004156 German.
-
Association of intravitreal and topical anti-inflammatory therapies on short-term anatomical and functional outcomes following epiretinal membrane surgery.Acta Ophthalmol. 2025 Jun;103(4):416-422. doi: 10.1111/aos.17430. Epub 2024 Dec 20. Acta Ophthalmol. 2025. PMID: 39707151 Free PMC article.
-
Long-Acting Fluocinolone Acetonide Intravitreal Implant for Recurrent Bilateral Non-Infectious Posterior Uveitis.Int Med Case Rep J. 2022 Nov 22;15:665-669. doi: 10.2147/IMCRJ.S384356. eCollection 2022. Int Med Case Rep J. 2022. PMID: 36444172 Free PMC article.
-
Comparative study of pars plana vitrectomy with or without intravitreal dexamethasone implant for idiopathic epiretinal membrane.Indian J Ophthalmol. 2020 Jun;68(6):1103-1107. doi: 10.4103/ijo.IJO_1045_19. Indian J Ophthalmol. 2020. PMID: 32461441 Free PMC article. Clinical Trial.
References
-
- Leopold IH. Nonsteroidal and steroidal anti-inflammatory agents. In: Sears ML, Tarkkanen A, editors. Surgical Pharmacology of the Eye. New York: Raven Press; 1985.
-
- Pagano G, Bruno A, Cavallo-Perin P, Cesco L, Imbimbo B. Glucose intolerance after short-term administration of corticosteroids in healthy subjects. Prednisone, deflazacort, and betamethasone. Arch Intern Med. 1989;149(5):1098–1101. - PubMed
-
- Saag KG. Glucocorticosteroid-induced osteoporosis. Endocrinol Metab Clin North Am. 2003;32(1):135–157. - PubMed
-
- Weijtens O, van der Sluijs FA, Schoemaker RC, et al. Peribulbar corticosteroid injection: vitreal and serum concentration after dexamethasone disodium phosphate injection. Am J Ophthalmol. 1997;123(3):358–363. - PubMed
