Structural basis for assembly and function of the Nup82 complex in the nuclear pore scaffold
- PMID: 25646085
- PMCID: PMC4315244
- DOI: 10.1083/jcb.201411003
Structural basis for assembly and function of the Nup82 complex in the nuclear pore scaffold
Abstract
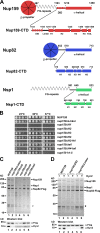
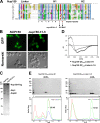
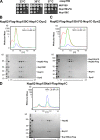
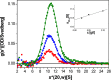
Nuclear pore complexes (NPCs) are huge assemblies formed from ∼30 different nucleoporins, typically organized in subcomplexes. One module, the conserved Nup82 complex at the cytoplasmic face of NPCs, is crucial to terminate mRNA export. To gain insight into the structure, assembly, and function of the cytoplasmic pore filaments, we reconstituted in yeast the Nup82-Nup159-Nsp1-Dyn2 complex, which was suitable for biochemical, biophysical, and electron microscopy analyses. Our integrative approach revealed that the yeast Nup82 complex forms an unusual asymmetric structure with a dimeric array of subunits. Based on all these data, we developed a three-dimensional structural model of the Nup82 complex that depicts how this module might be anchored to the NPC scaffold and concomitantly can interact with the soluble nucleocytoplasmic transport machinery.
© 2015 Gaik et al.
Figures



Similar articles
-
Structure of a yeast Dyn2-Nup159 complex and molecular basis for dynein light chain-nuclear pore interaction.J Biol Chem. 2012 May 4;287(19):15862-73. doi: 10.1074/jbc.M111.336172. Epub 2012 Mar 12. J Biol Chem. 2012. PMID: 22411995 Free PMC article.
-
Molecular basis for the functional interaction of dynein light chain with the nuclear-pore complex.Nat Cell Biol. 2007 Jul;9(7):788-96. doi: 10.1038/ncb1604. Epub 2007 Jun 3. Nat Cell Biol. 2007. PMID: 17546040
-
Structure and Function of the Nuclear Pore Complex Cytoplasmic mRNA Export Platform.Cell. 2016 Nov 17;167(5):1215-1228.e25. doi: 10.1016/j.cell.2016.10.028. Epub 2016 Nov 10. Cell. 2016. PMID: 27839866 Free PMC article.
-
[Nuclear pores: from yeast to higher eukaryotes].J Soc Biol. 2002;196(4):349-54. J Soc Biol. 2002. PMID: 12645306 Review. French.
-
Toward the atomic structure of the nuclear pore complex: when top down meets bottom up.Nat Struct Mol Biol. 2016 Jul;23(7):624-30. doi: 10.1038/nsmb.3244. Epub 2016 Jun 6. Nat Struct Mol Biol. 2016. PMID: 27273515 Free PMC article. Review.
Cited by
-
Architecture of the cytoplasmic face of the nuclear pore.Science. 2022 Jun 10;376(6598):eabm9129. doi: 10.1126/science.abm9129. Epub 2022 Jun 10. Science. 2022. PMID: 35679405 Free PMC article.
-
Biallelic mutations in nucleoporin NUP88 cause lethal fetal akinesia deformation sequence.PLoS Genet. 2018 Dec 13;14(12):e1007845. doi: 10.1371/journal.pgen.1007845. eCollection 2018 Dec. PLoS Genet. 2018. PMID: 30543681 Free PMC article.
-
Comprehensive structure and functional adaptations of the yeast nuclear pore complex.Cell. 2022 Jan 20;185(2):361-378.e25. doi: 10.1016/j.cell.2021.12.015. Epub 2022 Jan 3. Cell. 2022. PMID: 34982960 Free PMC article.
-
Developing genetic tools to exploit Chaetomium thermophilum for biochemical analyses of eukaryotic macromolecular assemblies.Sci Rep. 2016 Feb 11;6:20937. doi: 10.1038/srep20937. Sci Rep. 2016. PMID: 26864114 Free PMC article.
-
Linker Nups connect the nuclear pore complex inner ring with the outer ring and transport channel.Nat Struct Mol Biol. 2015 Oct;22(10):774-81. doi: 10.1038/nsmb.3084. Epub 2015 Sep 7. Nat Struct Mol Biol. 2015. PMID: 26344569
References
-
- Bailer S.M., Balduf C., and Hurt E.. 2001. The Nsp1p carboxy-terminal domain is organized into functionally distinct coiled-coil regions required for assembly of nucleoporin subcomplexes and nucleocytoplasmic transport. Mol. Cell. Biol. 21:7944–7955 10.1128/MCB.21.23.7944-7955.2001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

