Axillary Ultrasound After Neoadjuvant Chemotherapy and Its Impact on Sentinel Lymph Node Surgery: Results From the American College of Surgeons Oncology Group Z1071 Trial (Alliance)
- PMID: 25646192
- PMCID: PMC4606058
- DOI: 10.1200/JCO.2014.57.8401
Axillary Ultrasound After Neoadjuvant Chemotherapy and Its Impact on Sentinel Lymph Node Surgery: Results From the American College of Surgeons Oncology Group Z1071 Trial (Alliance)
Abstract
Purpose: The American College of Surgeons Oncology Group Z1071 trial reported a 12.6% false-negative rate (FNR) for sentinel lymph node (SLN) surgery after neoadjuvant chemotherapy (NAC) in cN1 disease. Patients were not selected for surgery based on response, but a secondary end point was to determine whether axillary ultrasound (AUS) after NAC after fine-needle aspiration cytology can identify abnormal nodes and guide patient selection for SLN surgery.
Patients and methods: Patients with T0-4, N1-2, M0 breast cancer underwent AUS after neoadjuvant chemotherapy. AUS images were centrally reviewed and classified as normal or suspicious lymph nodes. AUS findings were tested for association with pathologic nodal status and SLN FNR. The impact of AUS results to select patients for SLN surgery to reduce the FNR was assessed.
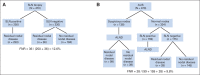
Results: Postchemotherapy AUS images were reviewed for 611 patients. One hundred thirty (71.8%) of 181 AUS-suspicious patients were node positive at surgery compared with 243 (56.5%) of 430 AUS-normal patients (P < .001). Patients with AUS-suspicious nodes had a greater number of positive nodes and greater metastasis size (P < .001). The SLN FNR was not different based on AUS results; however, using a strategy where only patients with normal AUS undergo SLN surgery would potentially reduce the FNR in Z1071 patients with ≥ two SLNs removed from 12.6% to 9.8% when preoperative AUS results are considered as part of SLN surgery.
Conclusion: AUS is recommended after chemotherapy to guide axillary surgery. An FNR of 9.8% with the combination of AUS and SLN surgery would be acceptable for the adoption of SLN surgery for women with node-positive breast cancer treated with neoadjuvant chemotherapy.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
Does a Picture Make a Difference? Ultrasound Guidance in the Management of the Axilla After Neoadjuvant Chemotherapy.J Clin Oncol. 2015 Oct 20;33(30):3367-9. doi: 10.1200/JCO.2014.60.1112. Epub 2015 Mar 2. J Clin Oncol. 2015. PMID: 25732160 No abstract available.
-
Sentinel Node Biopsy After Neoadjuvant Chemotherapy for Node-Positive Breast Cancer: Does Axillary Ultrasound Improve Performance?J Clin Oncol. 2015 Oct 20;33(30):3375-8. doi: 10.1200/JCO.2014.60.3316. Epub 2015 Sep 14. J Clin Oncol. 2015. PMID: 26371144 No abstract available.
References
-
- Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. - PubMed
-
- Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: Results from a prospective international cooperative group trial. Ann Surg Oncol. 2006;13:491–500. - PubMed
-
- Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–3663. - PubMed
-
- Hunt KK, Yi M, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009;250:558–566. - PubMed
-
- Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: Results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23:2694–2702. - PubMed
Publication types
MeSH terms
Grants and funding
- CA33601/CA/NCI NIH HHS/United States
- U10 CA76001/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180858/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- P30 CA015083/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA076001/CA/NCI NIH HHS/United States
- U10 CA180799/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

