An adjuvanted, tetravalent dengue virus purified inactivated vaccine candidate induces long-lasting and protective antibody responses against dengue challenge in rhesus macaques
- PMID: 25646261
- PMCID: PMC4385761
- DOI: 10.4269/ajtmh.14-0268
An adjuvanted, tetravalent dengue virus purified inactivated vaccine candidate induces long-lasting and protective antibody responses against dengue challenge in rhesus macaques
Abstract
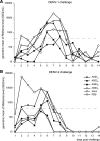
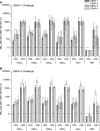
The immunogenicity and protective efficacy of a candidate tetravalent dengue virus purified inactivated vaccine (TDENV PIV) formulated with alum or an Adjuvant System (AS01, AS03 tested at three different dose levels, or AS04) was evaluated in a 0, 1-month vaccination schedule in rhesus macaques. One month after dose 2, all adjuvanted formulations elicited robust and persisting neutralizing antibody titers against all four dengue virus serotypes. Most of the formulations tested prevented viremia after challenge, with the dengue serotype 1 and 2 virus strains administered at 40 and 32 weeks post-dose 2, respectively. This study shows that inactivated dengue vaccines, when formulated with alum or an Adjuvant System, are candidates for further development.
© The American Society of Tropical Medicine and Hygiene.
Figures

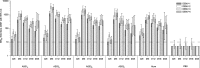
References
-
- Monath TP. Flaviviruses. In: Fields BN, Knipe DM, Chanock RM, editors. Field's Virology. 2nd Ed. New York, NY: Raven Press; 1990. pp. 763–814.
-
- Ye J, Zhu B, Fu ZF, Chen H, Cao S. Immune evasion strategies of flaviviruses. Vaccine. 2013;31:461–471. - PubMed
-
- Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005;3:13–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

